Reactivity 1.1 Measuring enthalpy changes
Reactivity 1.1.1
Understandings:
Understandings:
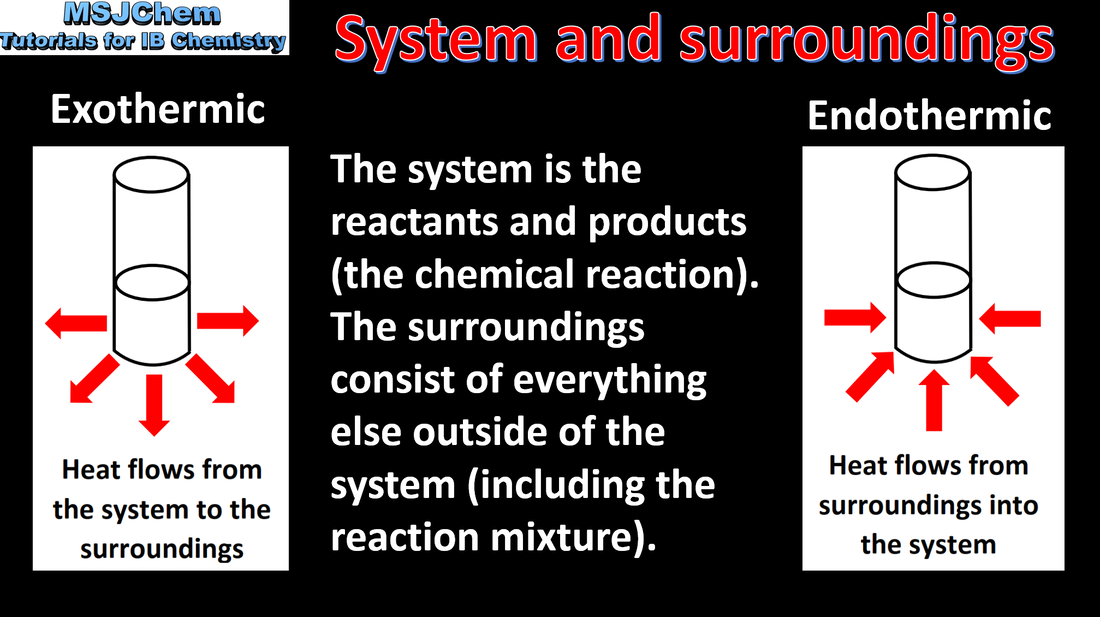
- Chemical reactions involve a transfer of energy between the system and the surroundings, while total energy is conserved.
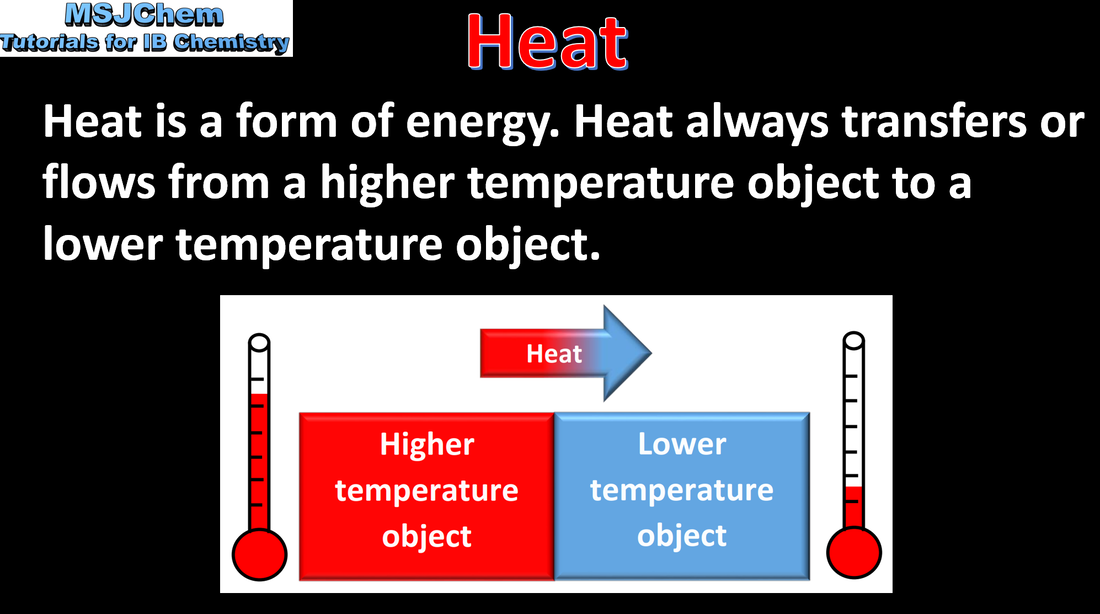
- Understand the difference between heat and temperature.
- Structure 1.1 What is the relationship between temperature and kinetic energy of particles?
Reactivity 1.1.2 and 1.1.3
Understandings:
Understandings:
- Reactions are described as endothermic or exothermic, depending on the direction of energy transfer between the system and the surroundings (1.1.2).
- The relative stability of reactants and products determines whether reactions are endothermic or exothermic (1.1.3).
- Understand the temperature change (decrease or increase) that accompanies endothermic and exothermic reactions, respectively (1.1.2).
- Sketch and interpret potential energy profiles for endothermic and exothermic reactions (1.1.3).
- Axes for energy profiles should be labelled as reaction coordinate (x) , potential energy (y) .
Reactivity 1.1.4
Understandings:
Understandings:
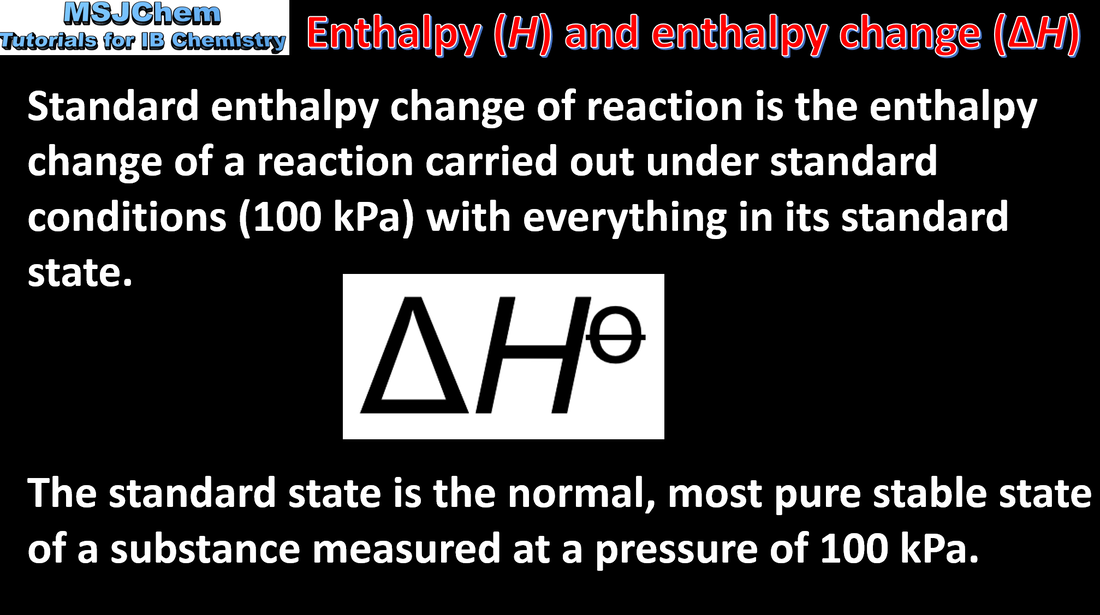
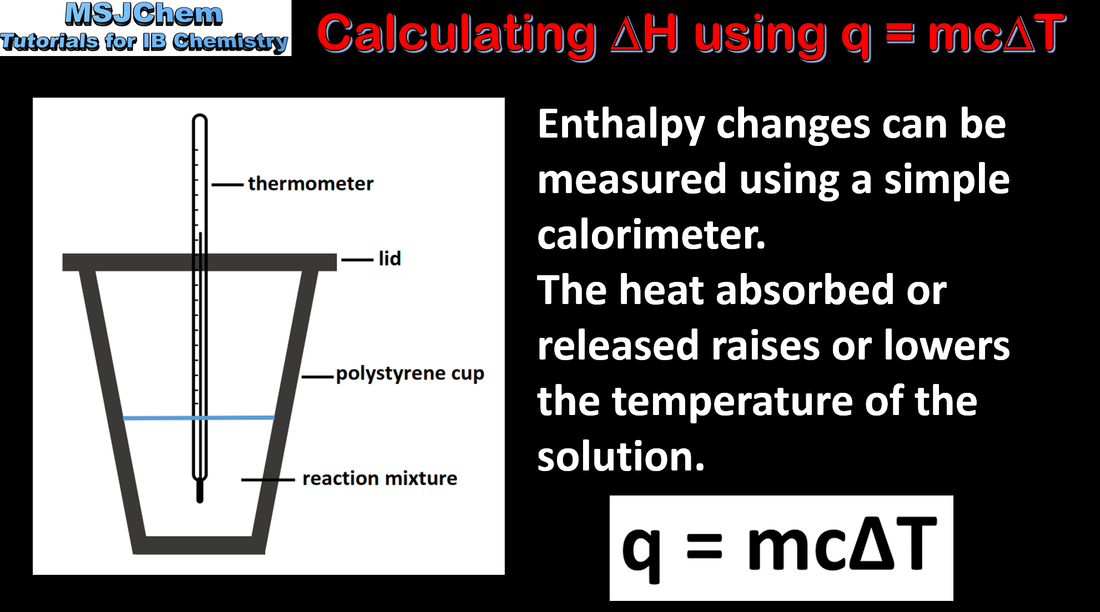
- The standard enthalpy change for a chemical reaction, ΔH⦵, refers to the heat transferred at constant pressure under standard conditions and states. It can be determined from the change in temperature of a pure substance.
- Apply the equations Q = mcΔT and ΔH = −Q/n in the calculation of the enthalpy change of a reaction.
- The units of ΔH⦵ are kJ mol–1.
- The equation Q = mcΔT and the value of c, the specific heat capacity of water, are given in the data booklet.