Reactivity 2.3 How far? The extent of chemical change (HL)
Reactivity 2.3.5
Understandings:
Understandings:
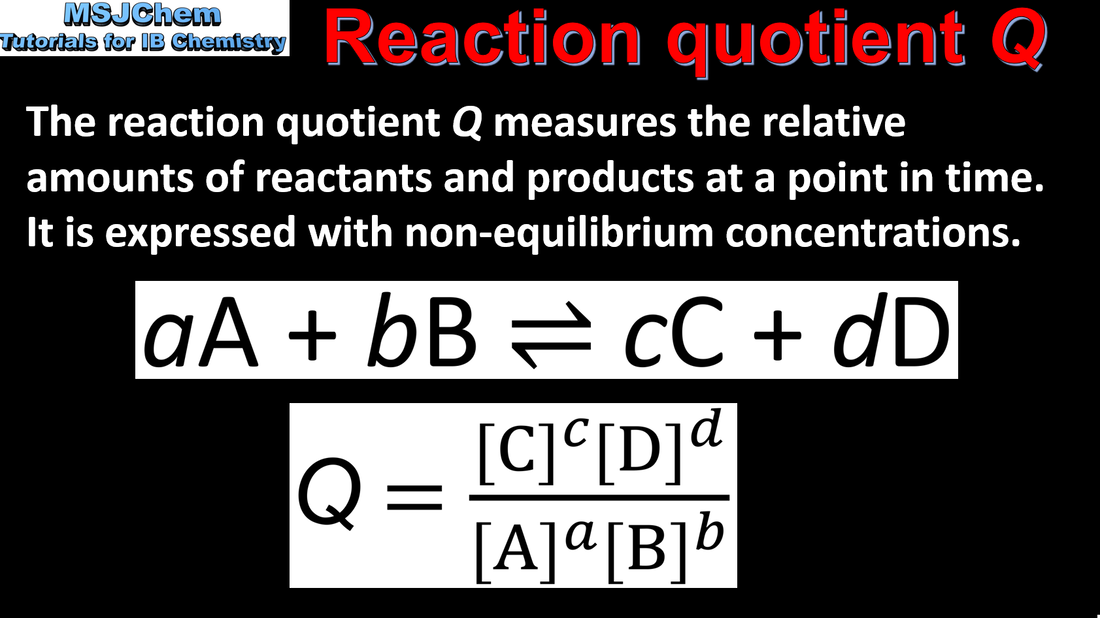
- The reaction quotient, Q, is calculated using the equilibrium expression with non- equilibrium concentrations of reactants and products.
- Calculate the reaction quotient Q from the concentrations of reactants and products at a particular time, and determine the direction in which the reaction will proceed to reach equilibrium.
Reactivity 2.3.6
Understandings:
Understandings:
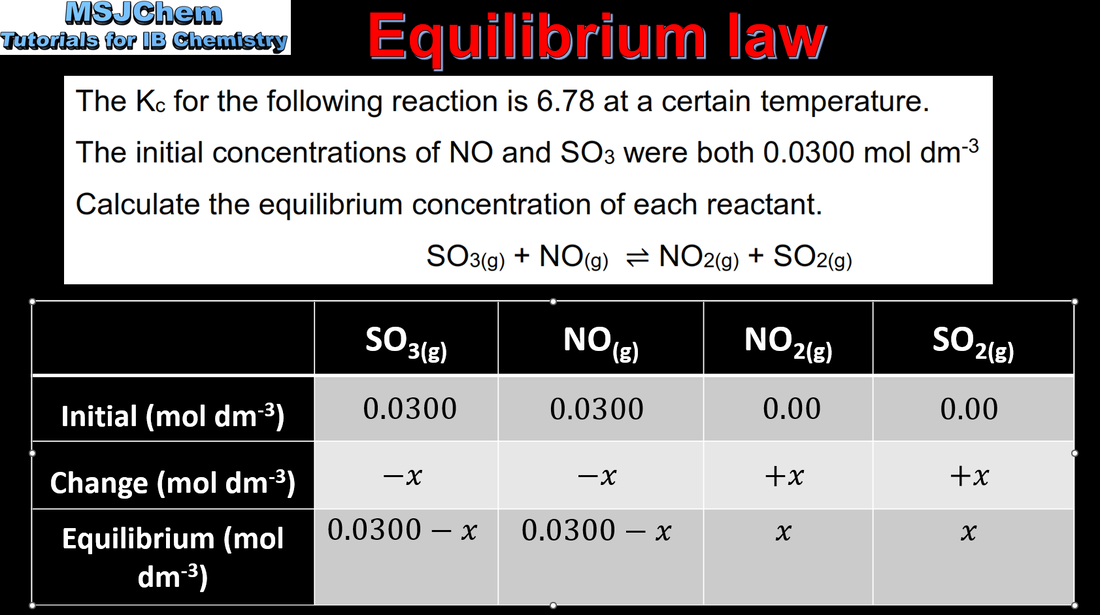
- The equilibrium law is the basis for quantifying the composition of an equilibrium mixture.
- Solve problems involving values of K and initial and equilibrium concentrations of the components of an equilibrium mixture.
- When K is very small, the approximation [reactant]initial ≈ [reactant]eqm should be understood.
- The use of quadratic equations is not expected. Only homogeneous equilibria will be assessed.
- Reactivity 3.1 How does the equilibrium law help us to determine the pH of a weak acid, weak base or a buffer solution?
Reactivity 2.3.7
Understandings:
Understandings:
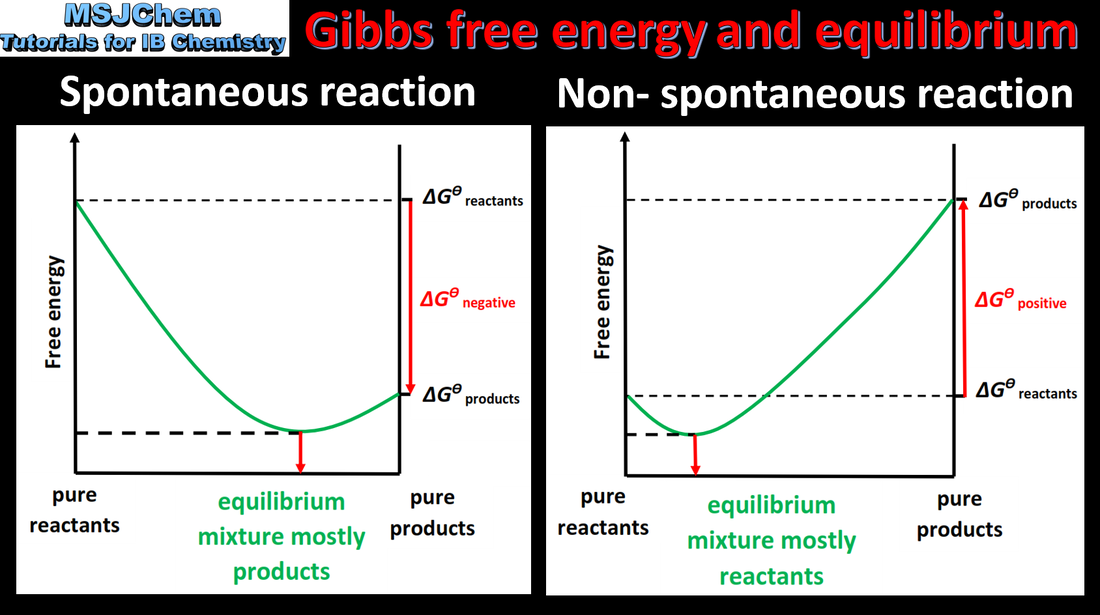
- The equilibrium constant and Gibbs energy change, ΔG, can both be used to measure the position of an equilibrium reaction.
- Calculations using the equation ΔG = −RT lnK.
- The equation is given in the data booklet.
- Reactivity 1.4 How can Gibbs energy be used to explain which of the forward or backward reaction is favoured before reaching equilibrium?