Help support my work by joining the Member's Area or by becoming a Patron.
Topic 4 Bonding
4.1 The octet rule
This video covers the relationship between the difference in electronegativity between atoms and the type of bonding.
Understandings:
Bond polarity results from the difference in electronegativities of the bonded atoms.
Understandings:
Bond polarity results from the difference in electronegativities of the bonded atoms.
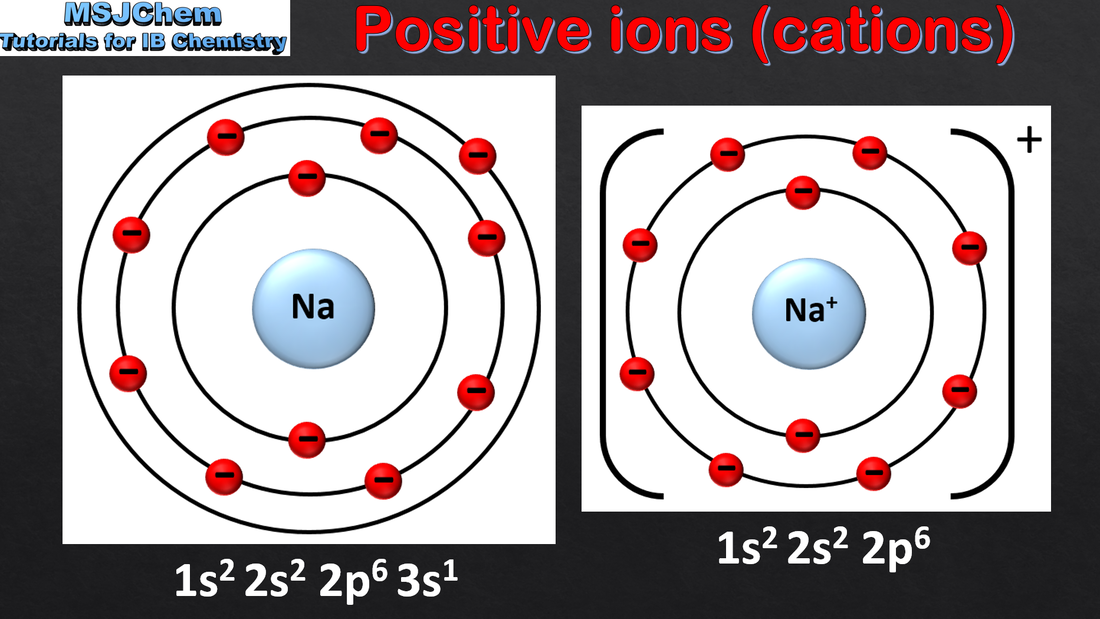
4.1 Ions and ion formation
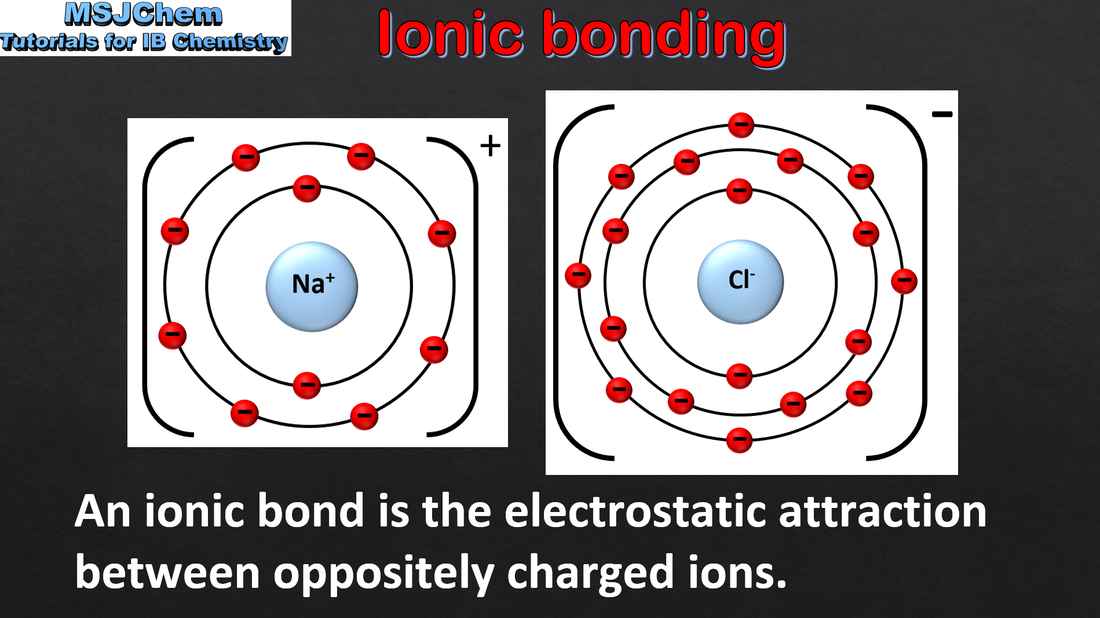
4.1 Ionic bonding
| topic_4_ionic_bonding.pdf |
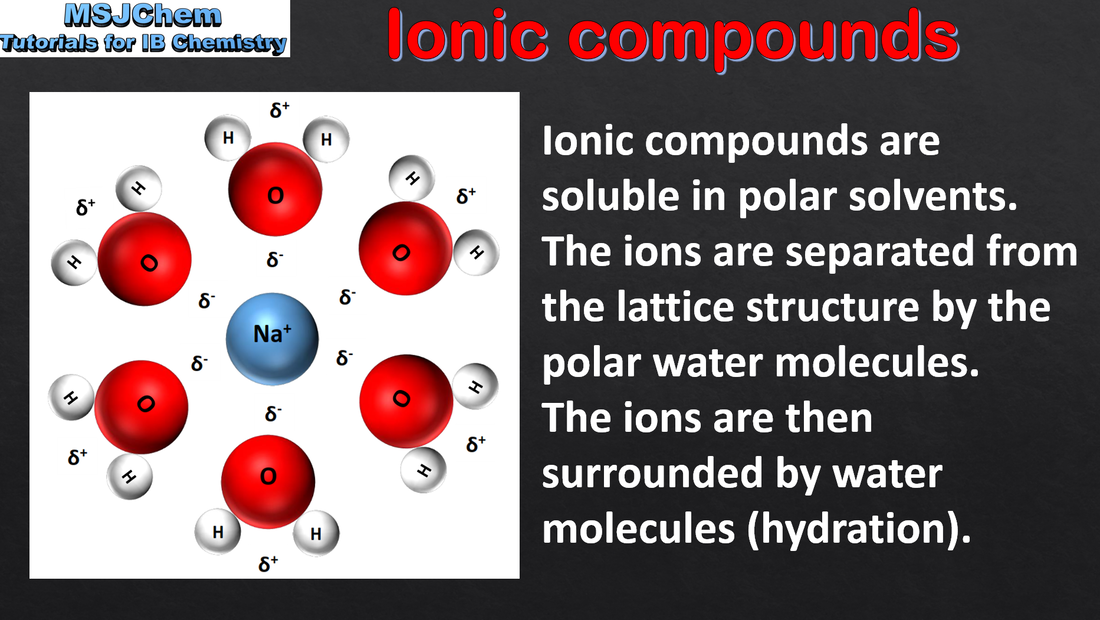
4.1 Structure and properties of ionic compounds
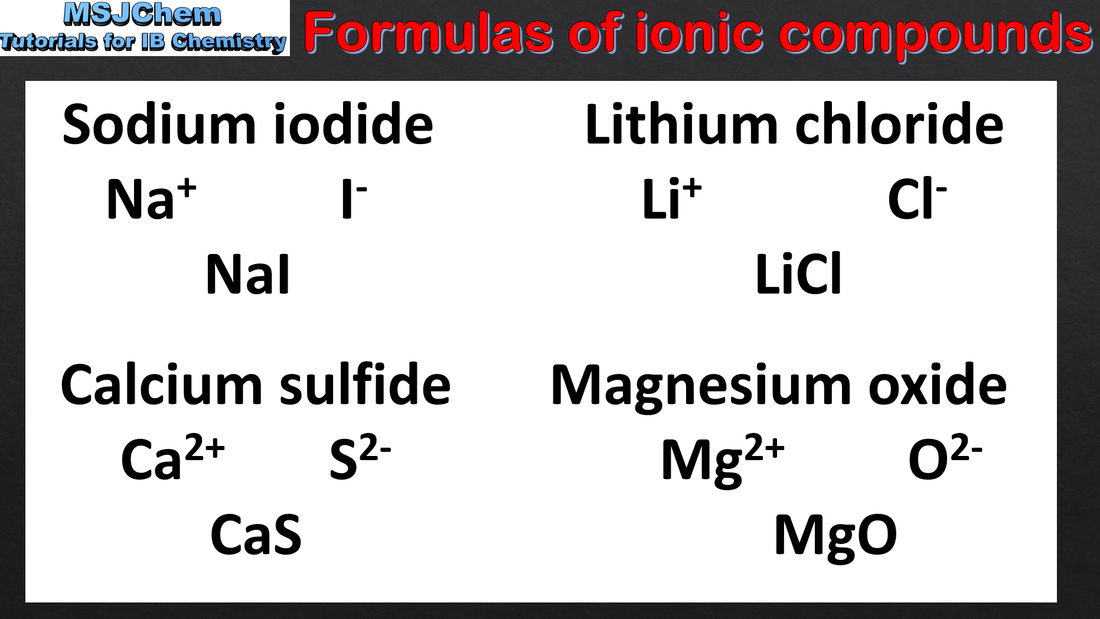
4.1 Writing formulae of ionic compounds
Applications and skills:
Deduction of the formula and name of an ionic compound from its component ions, including polyatomic ions.
Guidance:
Students should be familiar with the following polyatomic ions: NH4+ OH- HCO3- CO32- SO42- PO43- NO3-
Deduction of the formula and name of an ionic compound from its component ions, including polyatomic ions.
Guidance:
Students should be familiar with the following polyatomic ions: NH4+ OH- HCO3- CO32- SO42- PO43- NO3-
4.2 Covalent bonding
|
Understandings:
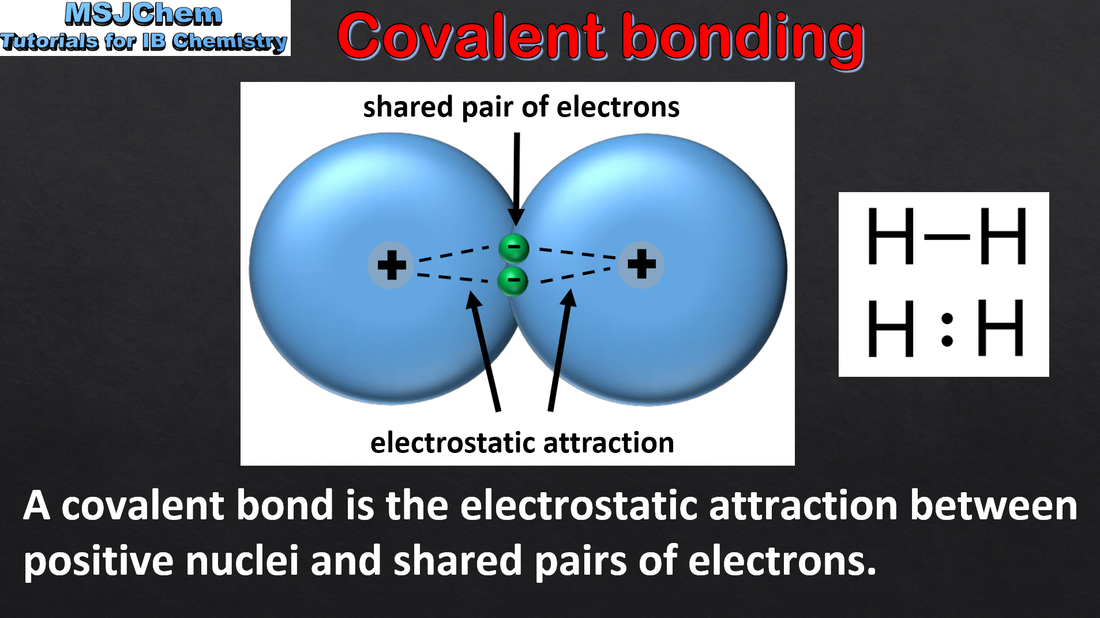
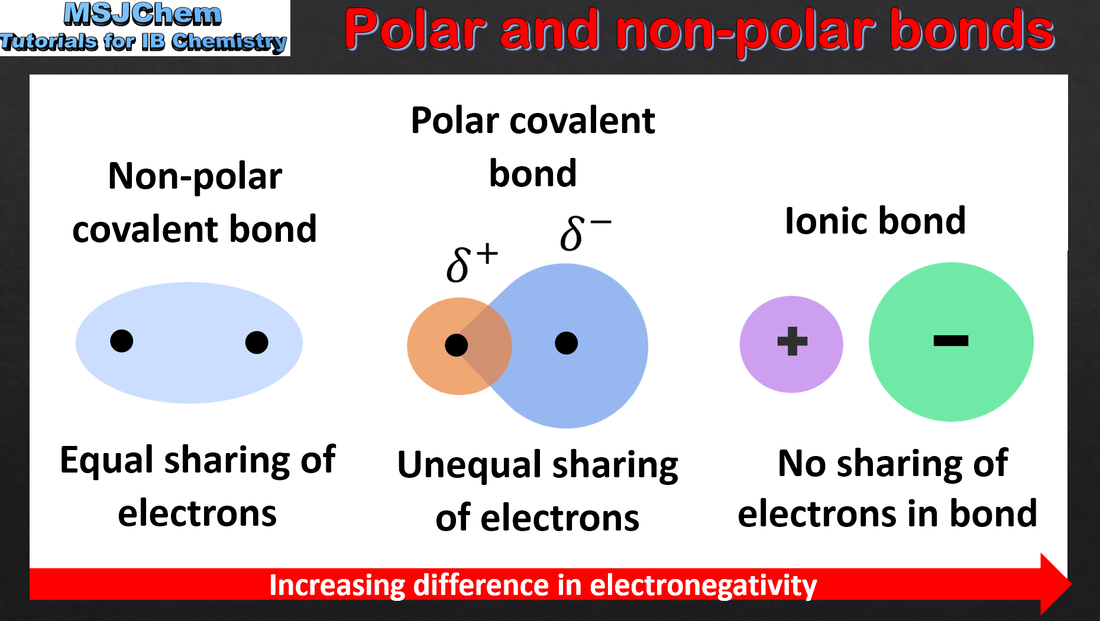
A covalent bond is formed by the electrostatic attraction between a shared pair of electrons and the positively charged nuclei. Bond polarity results from the difference in electronegativities of the bonded atoms. Single, double and triple covalent bonds involve one, two and three shared pairs of electrons respectively. Bond length decreases and bond strength increases as the number of shared electrons increases. Applications and skills: Deduction of the polar nature of a covalent bond from electronegativity values. |
| topic_4_covalent_bonding.pdf |
| topic_4_bond_polarity.pdf |
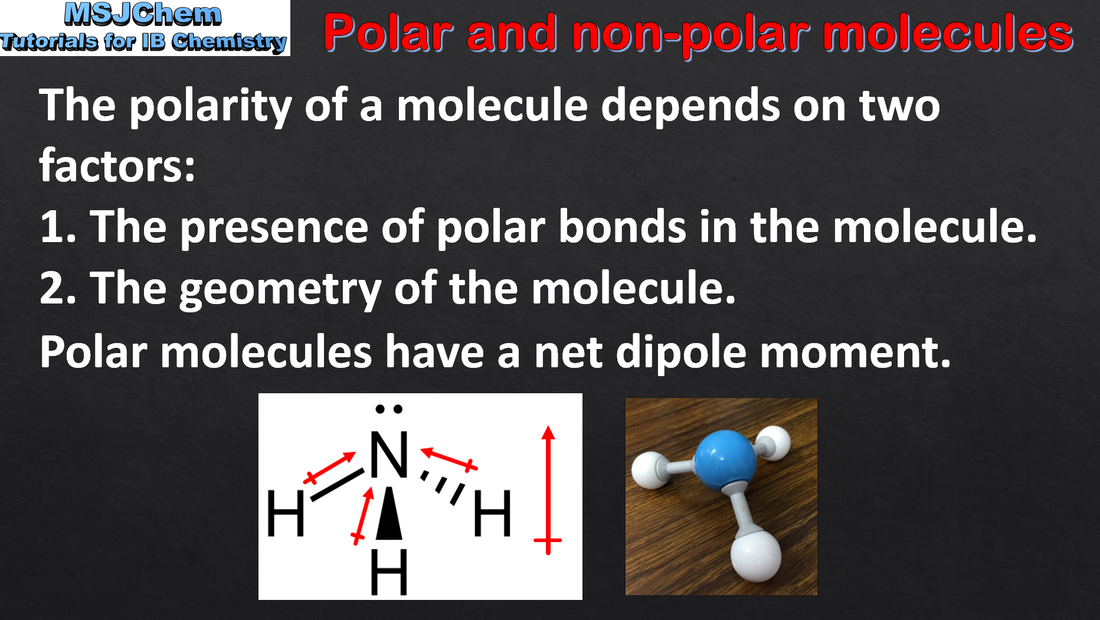
4.2 Polar and non-polar covalent bonds
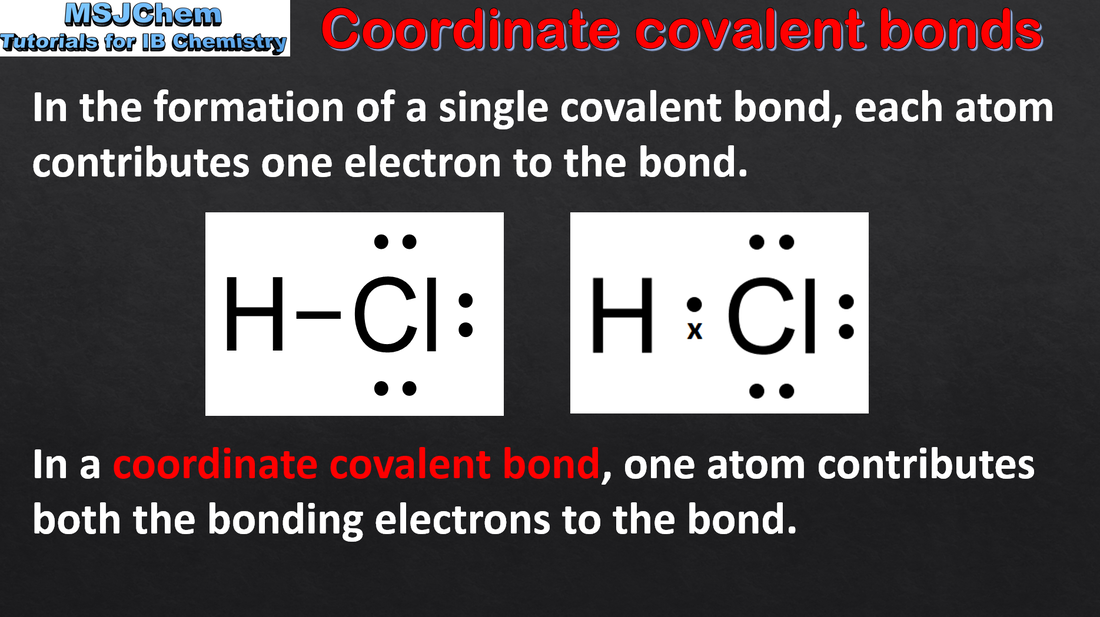
4.2 Coordinate covalent bonds
| topic_4_polar_and_non-polar_molecules.pdf |
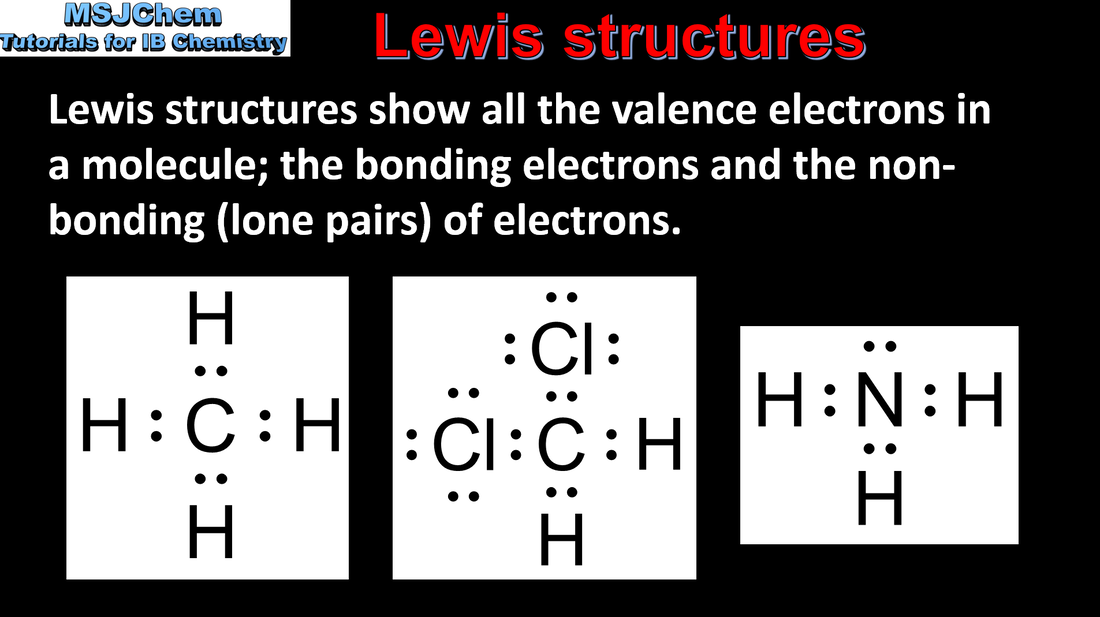
4.3 Lewis structures
|
Understandings:
Lewis (electron dot) structures show all the valence electrons in a covalently bonded species. The “octet rule” refers to the tendency of atoms to gain a valence shell with a total of 8 electrons. Some atoms, like Be and B, might form stable compounds with incomplete octets of electrons. Applications and skills: Deduction of Lewis (electron dot) structure of molecules and ions showing all valence electrons for up to four electron pairs on each atom. |
| topic_4_lewis_structures.pdf |
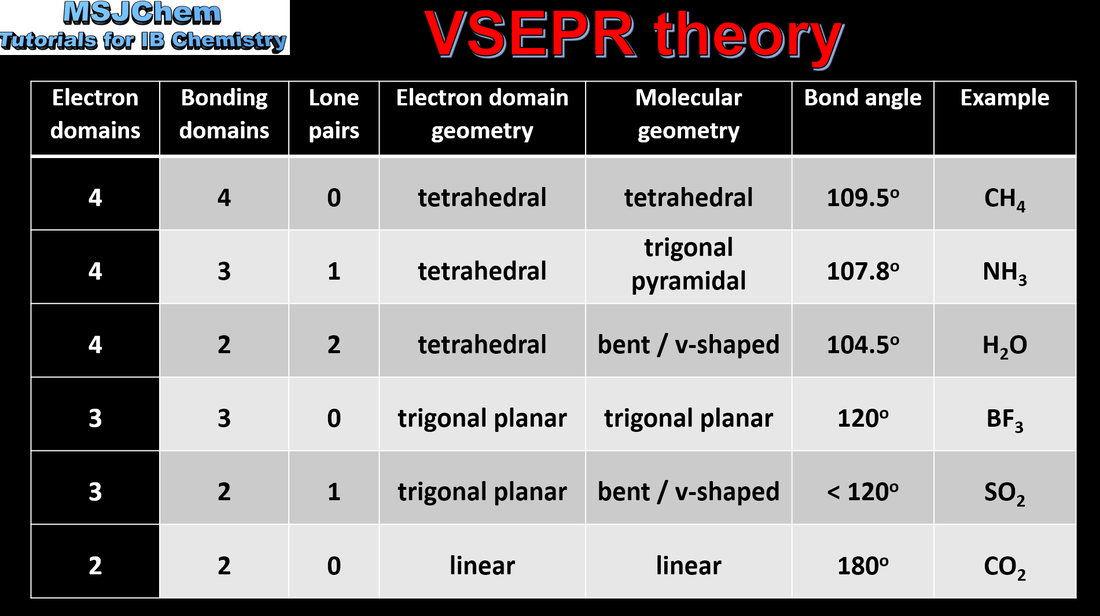
4.3 VSEPR theory
|
Understandings:
Shapes of species are determined by the repulsion of electron pairs according to VSEPR theory. Applications and skills: Deduction of Lewis (electron dot) structure of molecules and ions showing all valence electrons for up to four electron pairs on each atom. The use of VSEPR theory to predict the electron domain geometry and the molecular geometry for species with two, three and four electron domains. Prediction of bond angles from molecular geometry and presence of non- bonding pairs of electrons. |
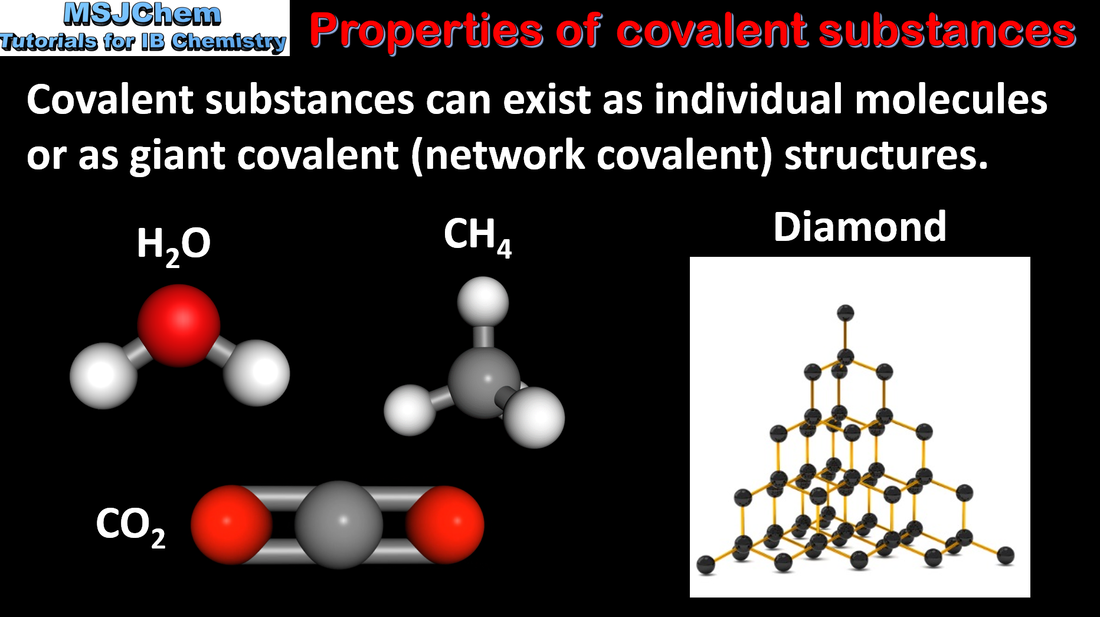
4.3 Structure and properties of covalent compounds
|
Understandings:
Carbon and silicon form giant covalent/network covalent structures. Application and skills: Explanation of the properties of giant covalent compounds in terms of their structures. Explanation of the physical properties of covalent compounds (volatility, electrical conductivity and solubility) in terms of their structure and intermolecular forces. |
| topic_4_structure_and_properties_of_covalent_compounds.pdf |
4.3 Allotropes of carbon
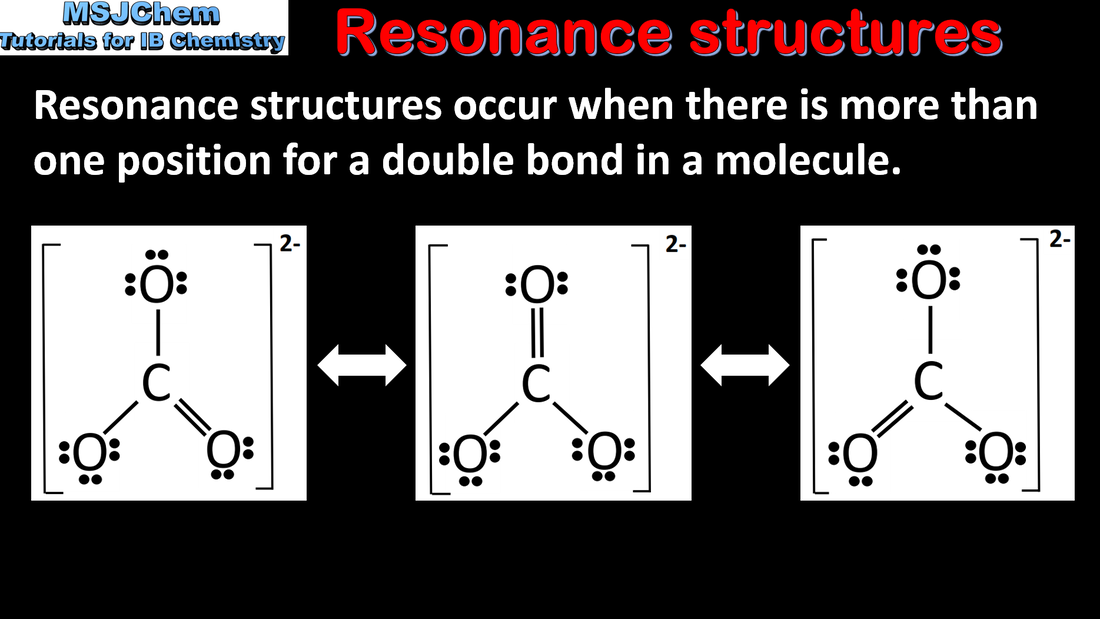
4.3 Resonance structures
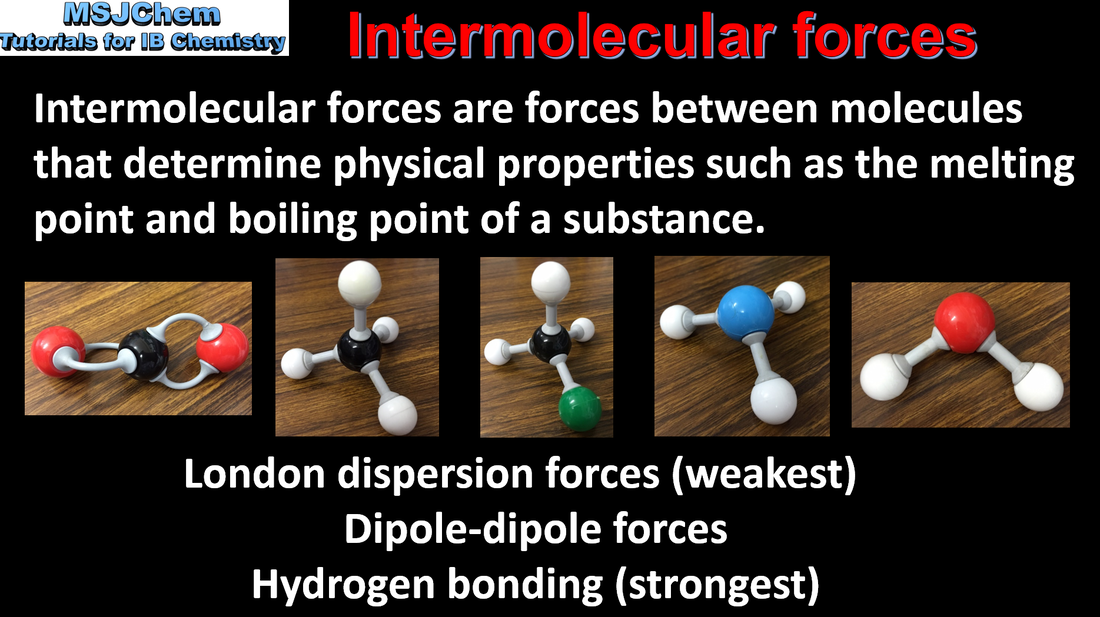
4.4 Intermolecular forces
|
Understandings:
Intermolecular forces include London (dispersion) forces, dipole-dipole forces and hydrogen bonding. The relative strengths of these interactions are London (dispersion) forces < dipole-dipole forces < hydrogen bonds. Applications and skills: Deduction of the types of intermolecular force present in substances, based on their structure and chemical formula. |
Note: The term van der Waals forces includes London dispersion forces (instantaneous and induced dipoles) and dipole-dipole forces.
| topic_4_intermolecular_forces.pdf |
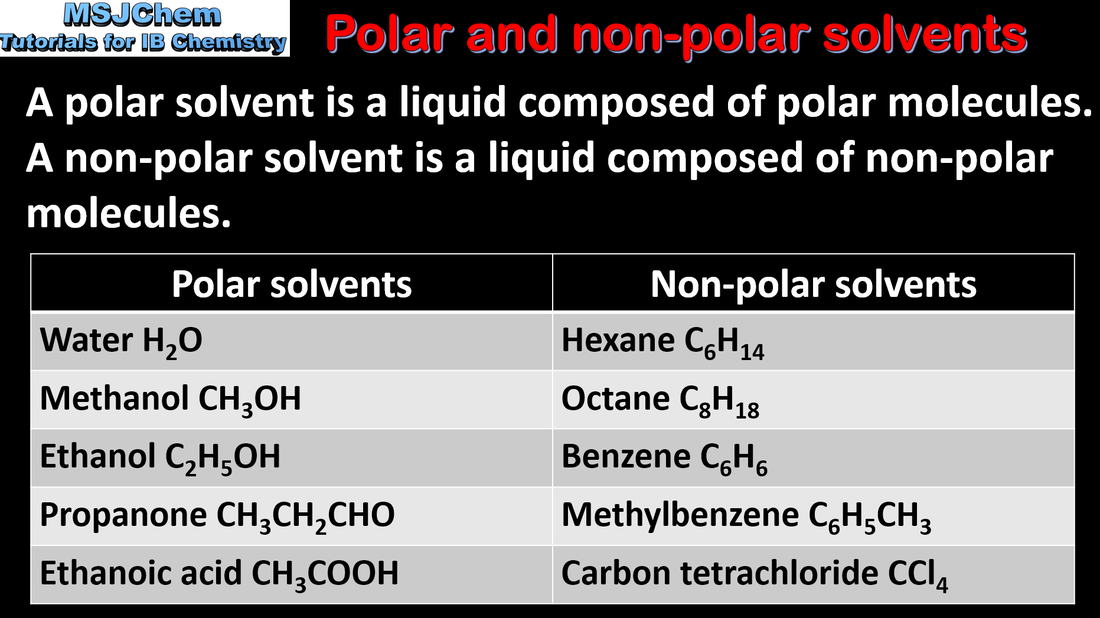
4.4 Solubility and intermolecular forces
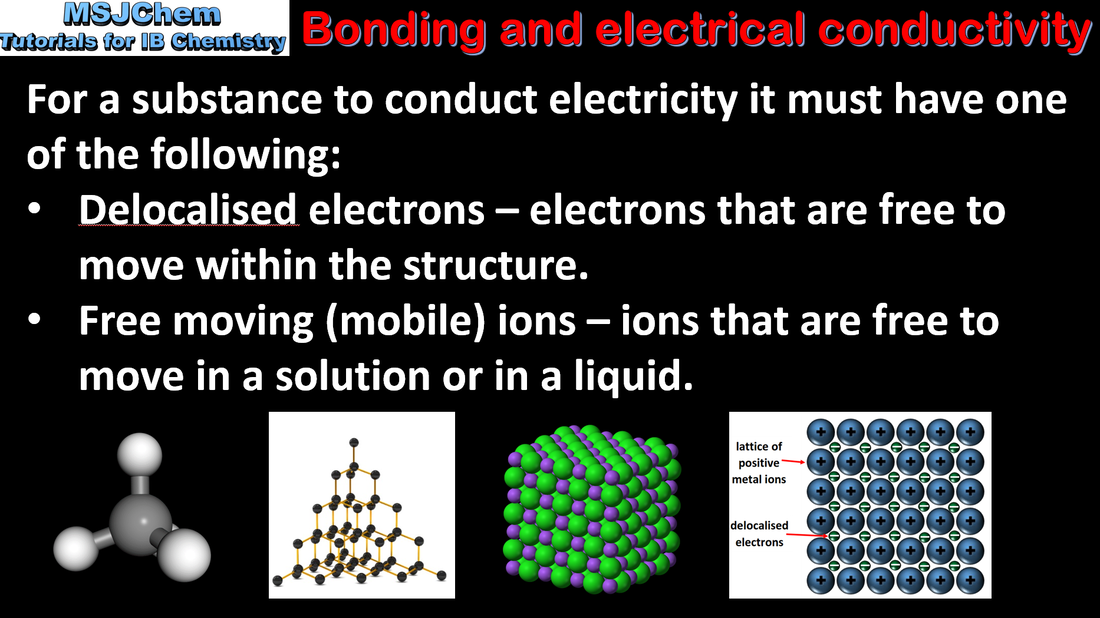
Types of bonding and electrical conductivity
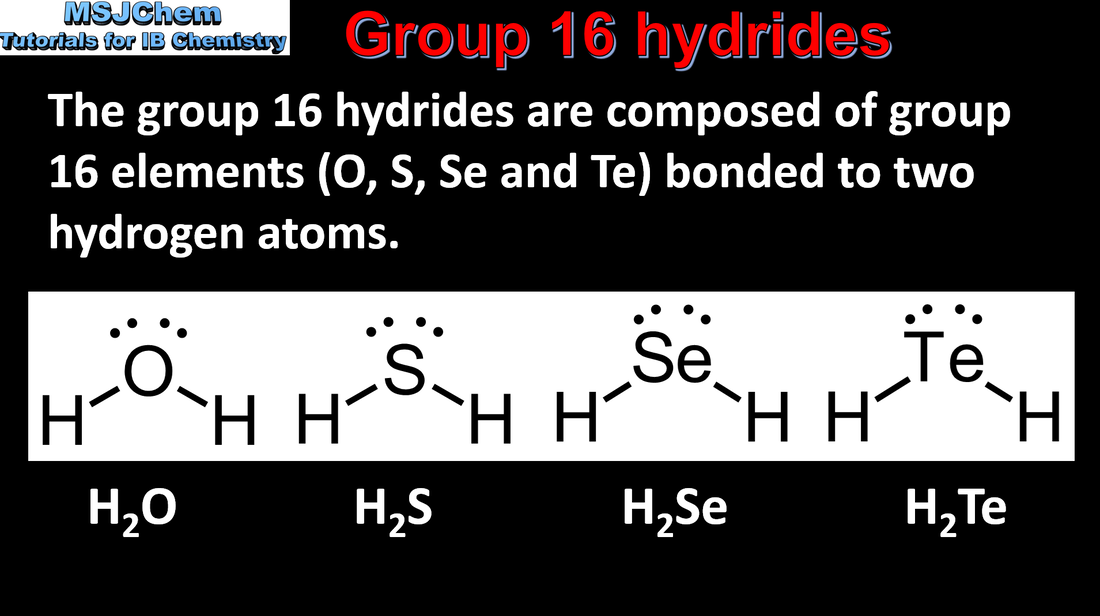
4.4 Group 16 hydrides
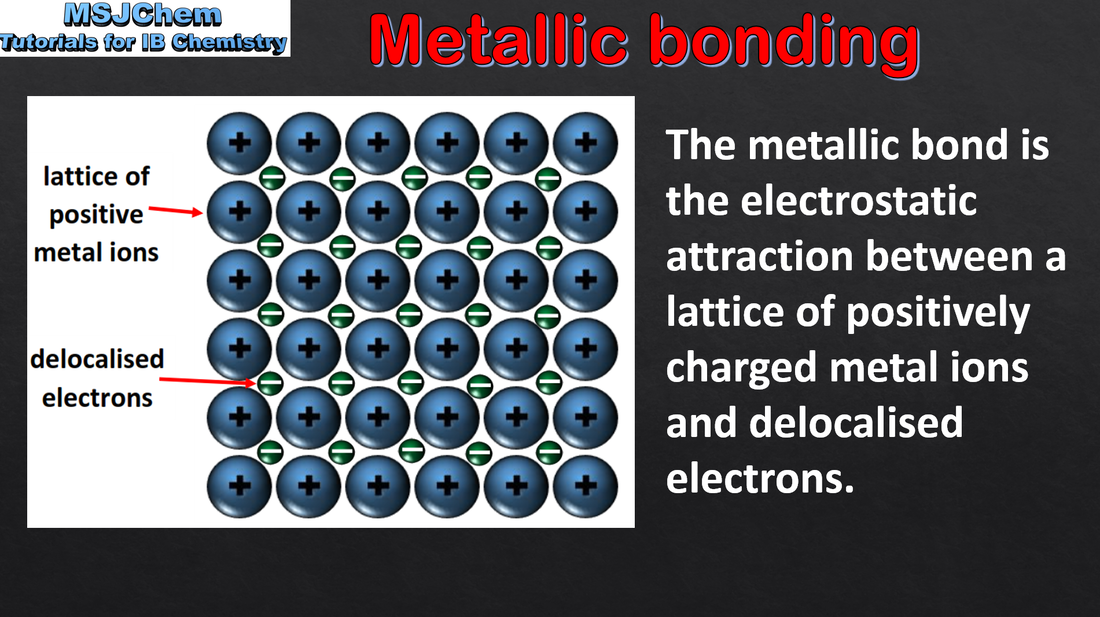
4.5 Metallic bonding
|
Understandings:
A metallic bond is the electrostatic attraction between a lattice of positive ions and delocalized electrons. The strength of a metallic bond depends on the charge of the ions and the radius of the metal ion. Applications and skills: Explanation of electrical conductivity and malleability in metals. Explanation of trends in melting points of metals. |
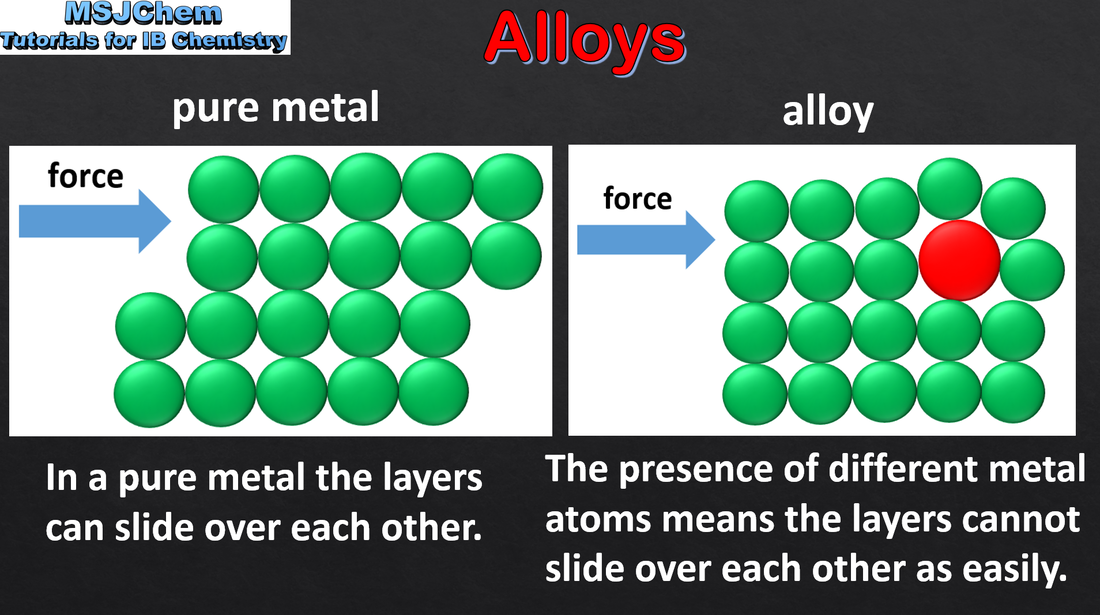
4.5 Alloys