Help support my work by joining the Member's Area or by becoming a Patron.
Topic 7 Equilibrium
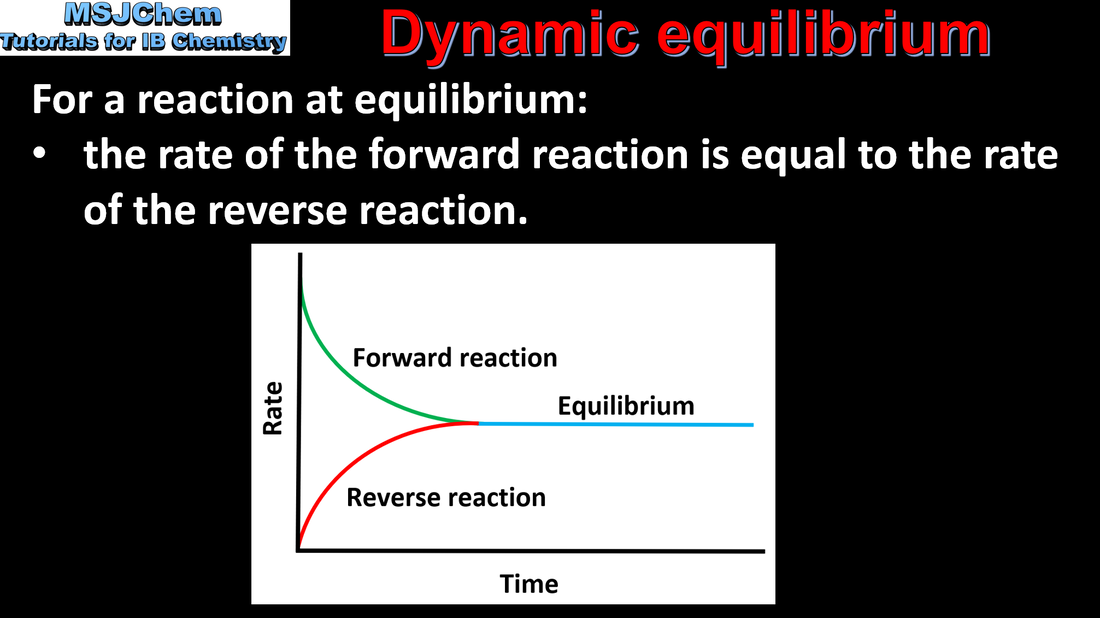
7.1 Dynamic equilibrium
7.1 Physical equilibrium
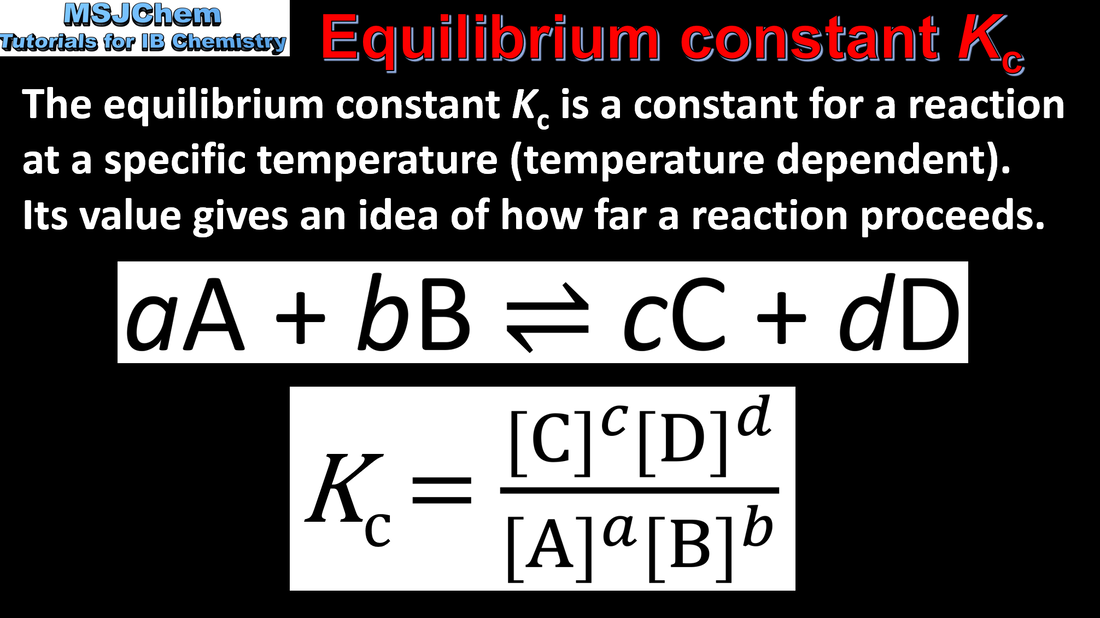
7.1 Equilibrium constant Kc.
|
Understandings:
The equilibrium law describes how the equilibrium constant (Kc) can be determined for a particular chemical reaction. The magnitude of the equilibrium constant indicates the extent of a reaction at equilibrium and is temperature dependent. Applications and skills: Deduction of the equilibrium constant expression (Kc) from an equation for a homogeneous reaction. |
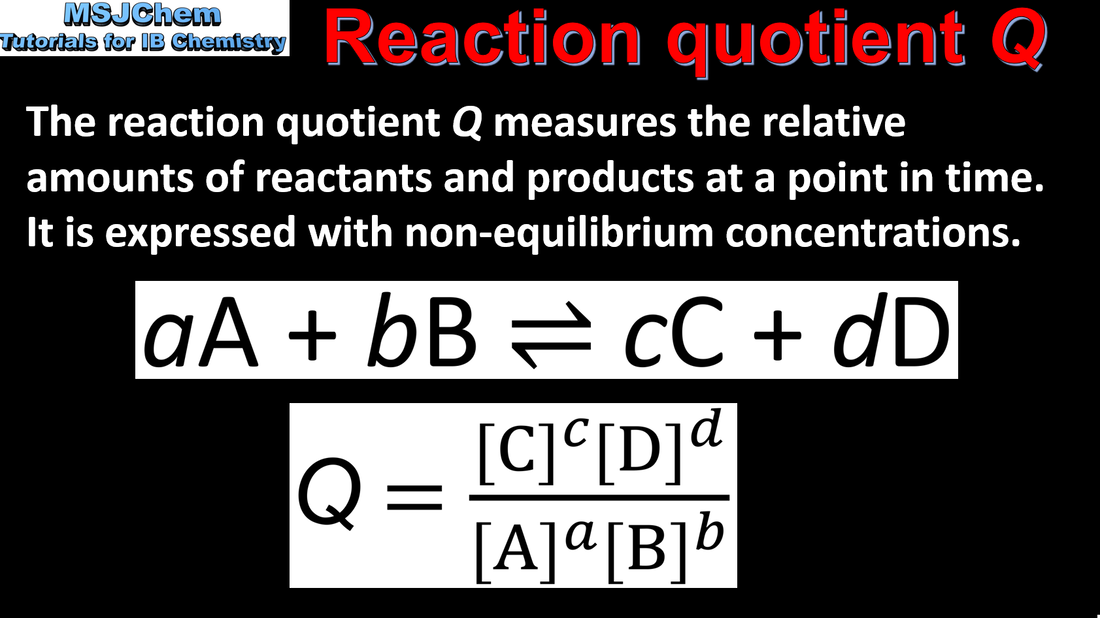
7.1 Reaction quotient Q
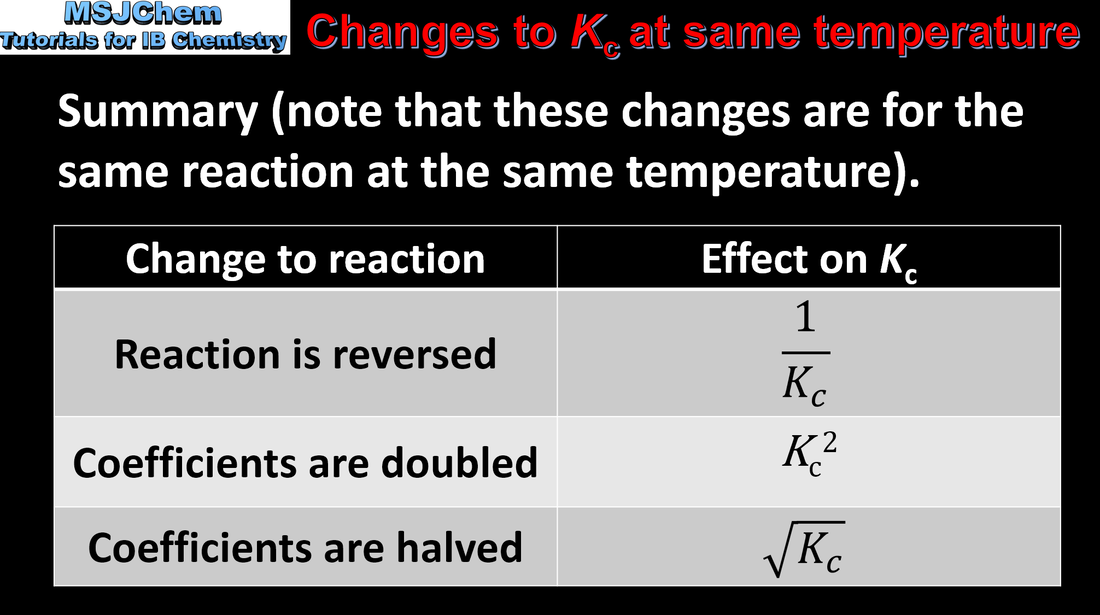
7.1 Manipulating Kc
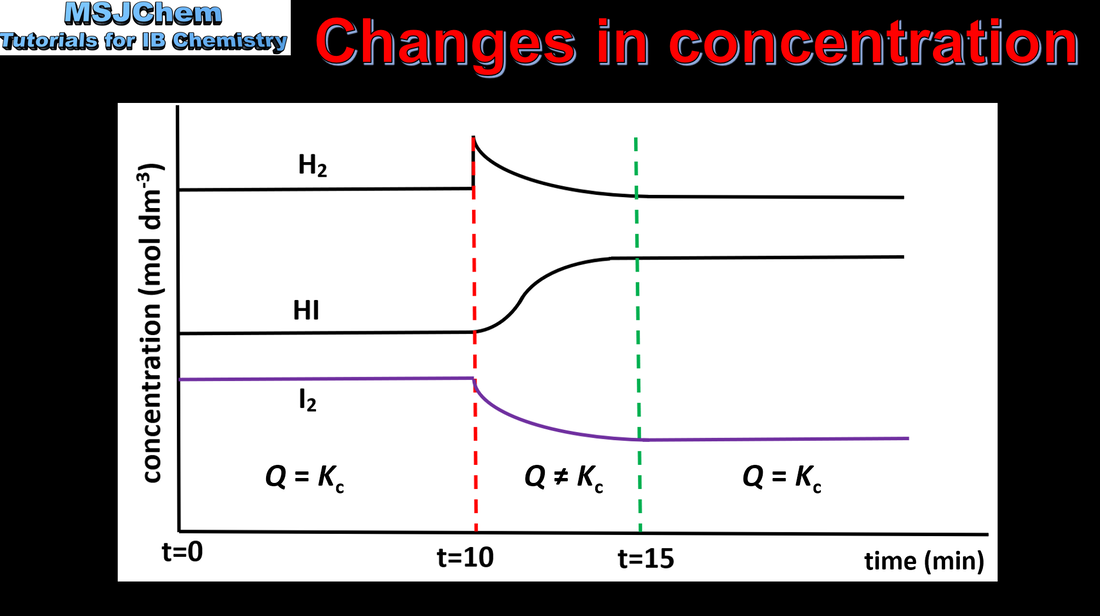
7.1 Le Chatelier's principle (changes in concentration)
|
Applications and skills:
Application of Le Châtelier’s principle to predict the qualitative effects of changes of temperature, pressure and concentration on the position of equilibrium and on the value of the equilibrium constant. This video covers changes in concentration. The next video covers changes in pressure. |
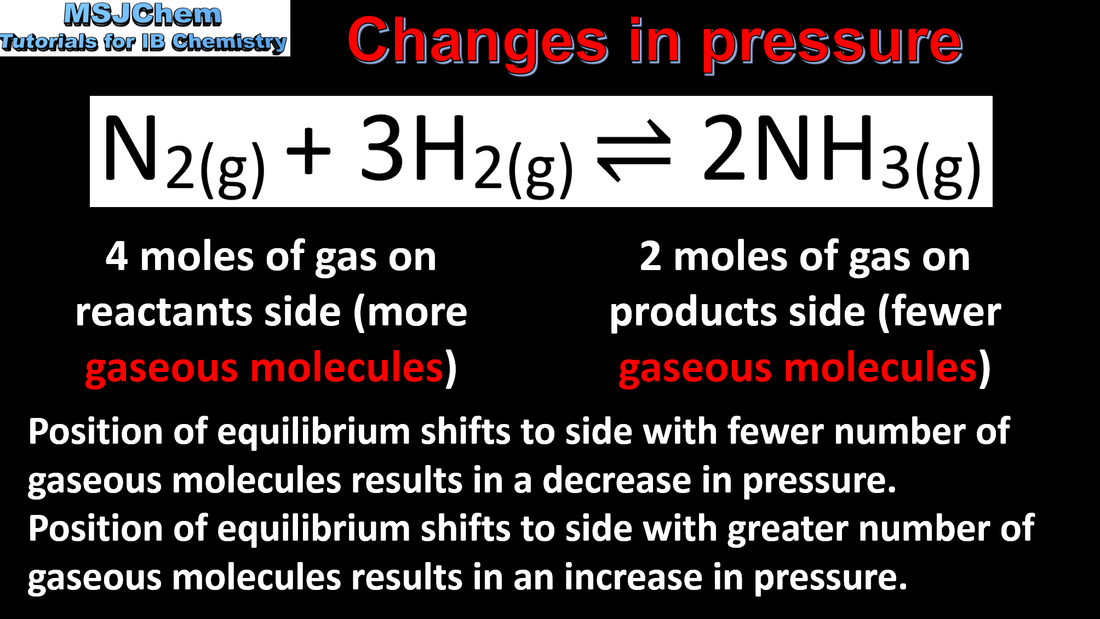
7.1 Le Chatelier's principle (changes in pressure)
|
Applications and skills:
Application of Le Châtelier’s principle to predict the qualitative effects of changes of temperature, pressure and concentration on the position of equilibrium and on the value of the equilibrium constant. This video covers changes in pressure. The next video covers changes in temperature. |
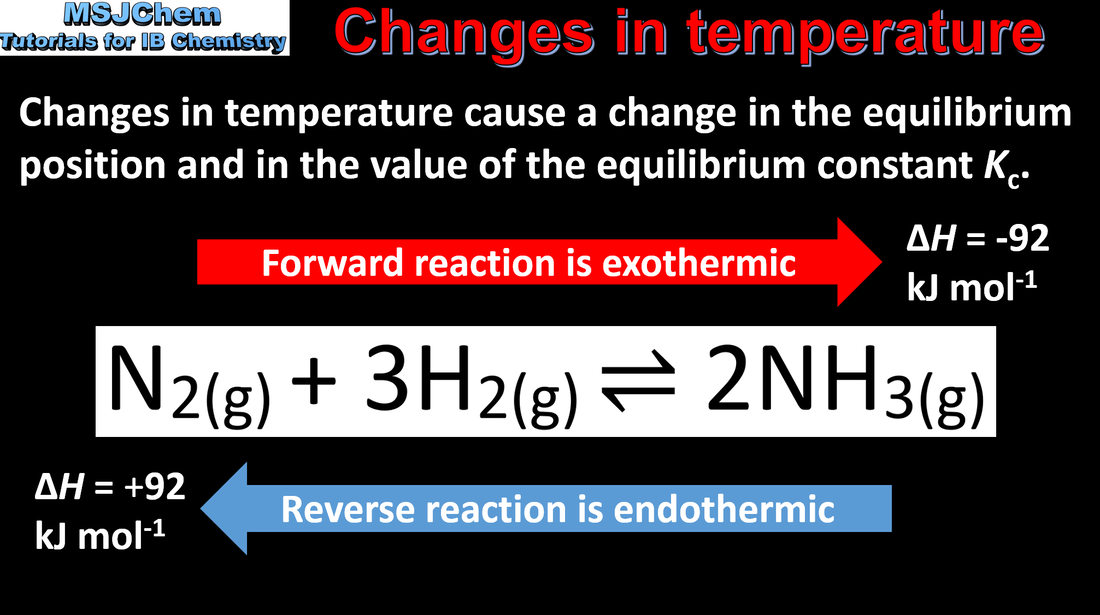
7.1 Le Chatelier's principle (changes in temperature)
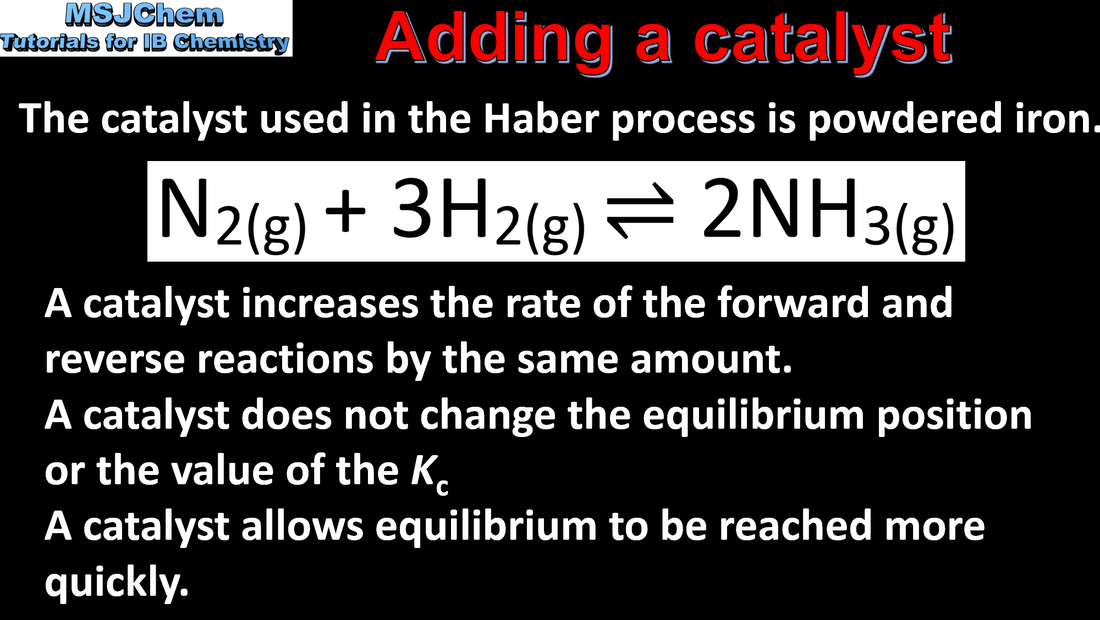
7.1 The effect of a catalyst on an equilibrium reaction.