Topic 15 Energetics HL
YouTube playlist
YouTube playlist
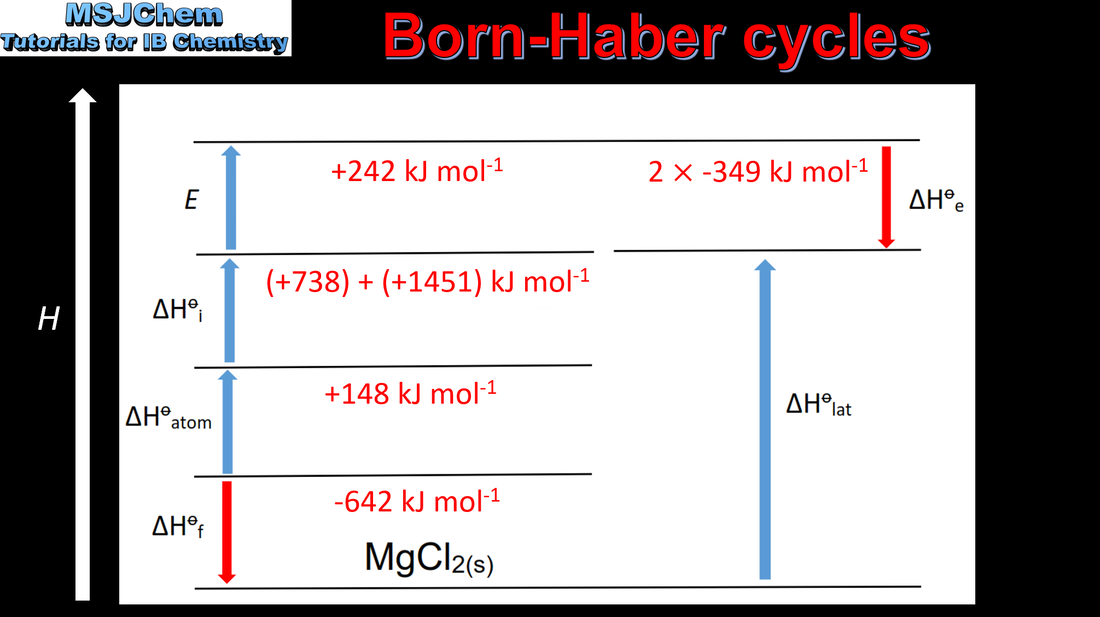
15.1 Born Haber cycles
|
|
Applications and skills:
Relate size and charge of ions to lattice and hydration enthalpies. |
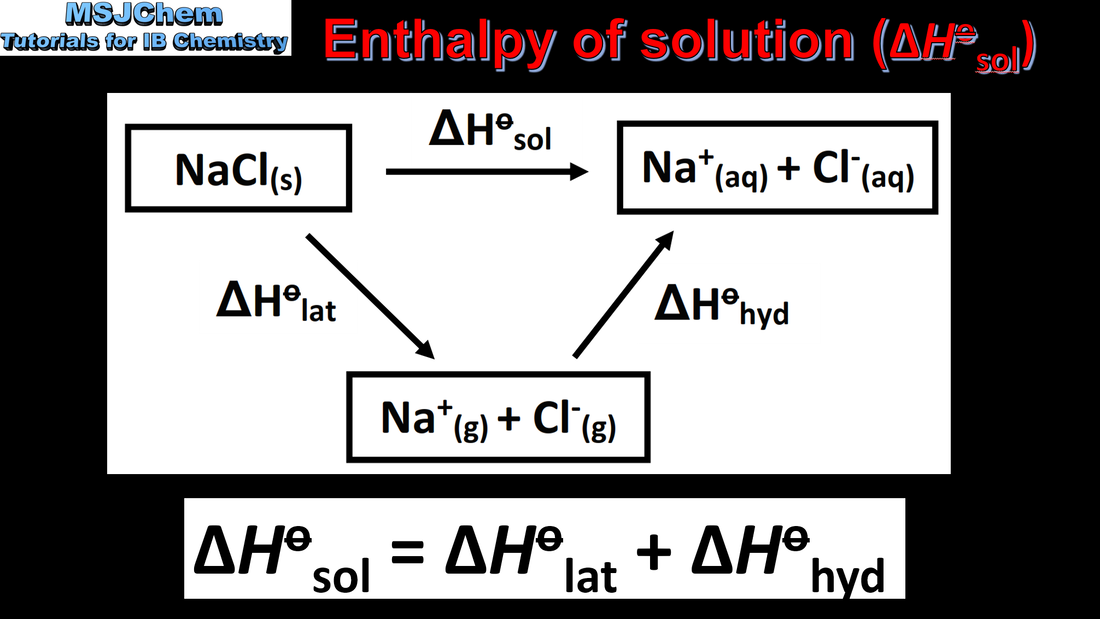
15.1 Enthalpy change of solution and hydration
|
|
This video covers the standard enthalpy changes of formation and combustion.
Please note that the standard conditions are now 298 K and 100.0 kPa. |
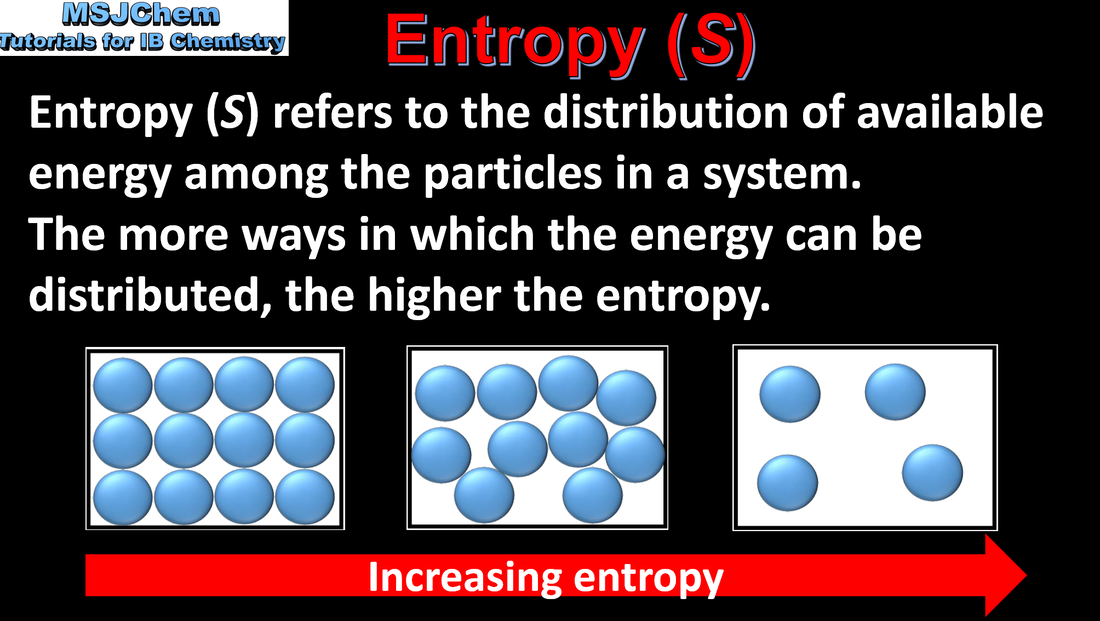
15.2 Entropy
|
Understandings:
Entropy (S) refers to the distribution of available energy among the particles. The more ways the energy can be distributed the higher the entropy. Order of increasing entropy: solids - liquids - gases Note that IB students are not required to use the equation shown in the video to calculate entropy. |
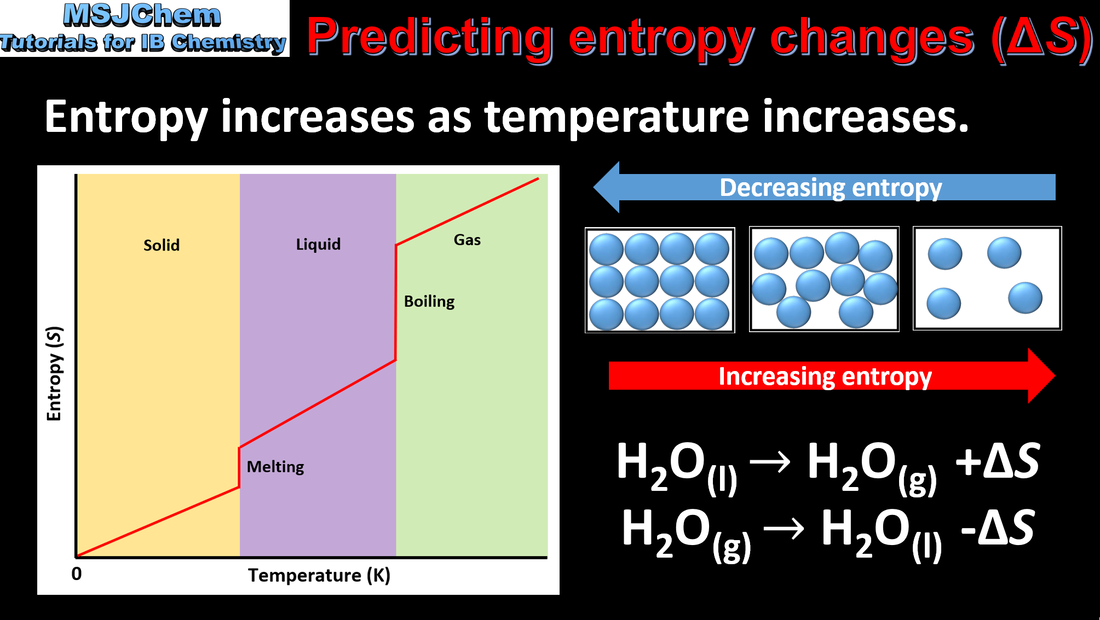
15.2 Predicting entropy changes
|
Understandings:
Entropy (S) refers to the distribution of available energy among the particles. The more ways the energy can be distributed the higher the entropy. Entropy of gas>liquid>solid under same conditions. Application and skills: Prediction of whether a change will result in an increase or decrease in entropy by considering the states of the reactants and products. |
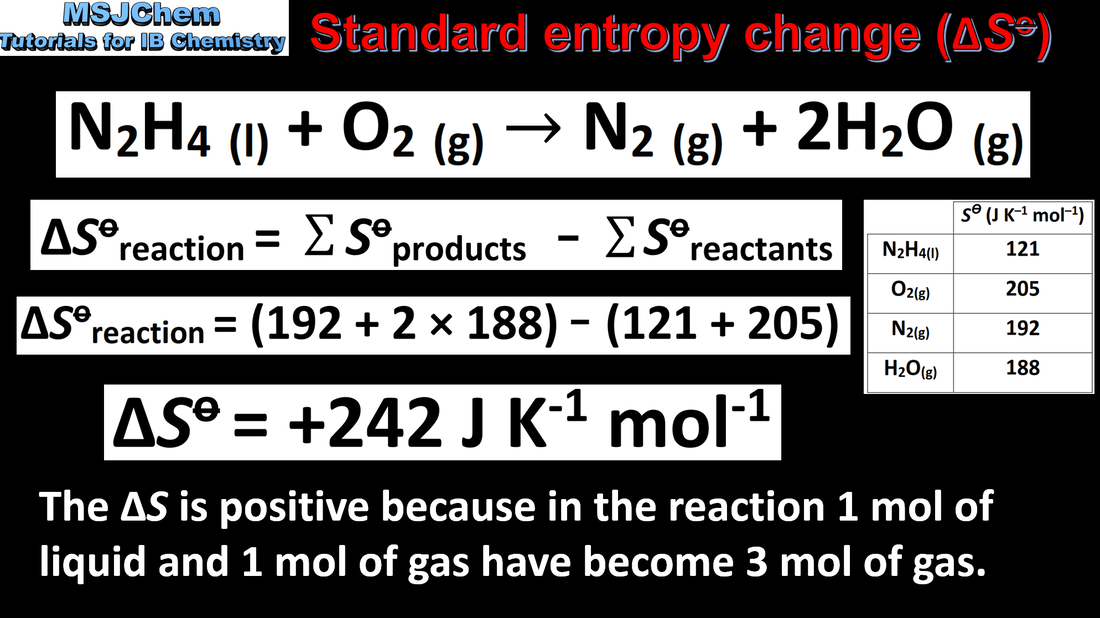
15.2 Calculating entropy changes
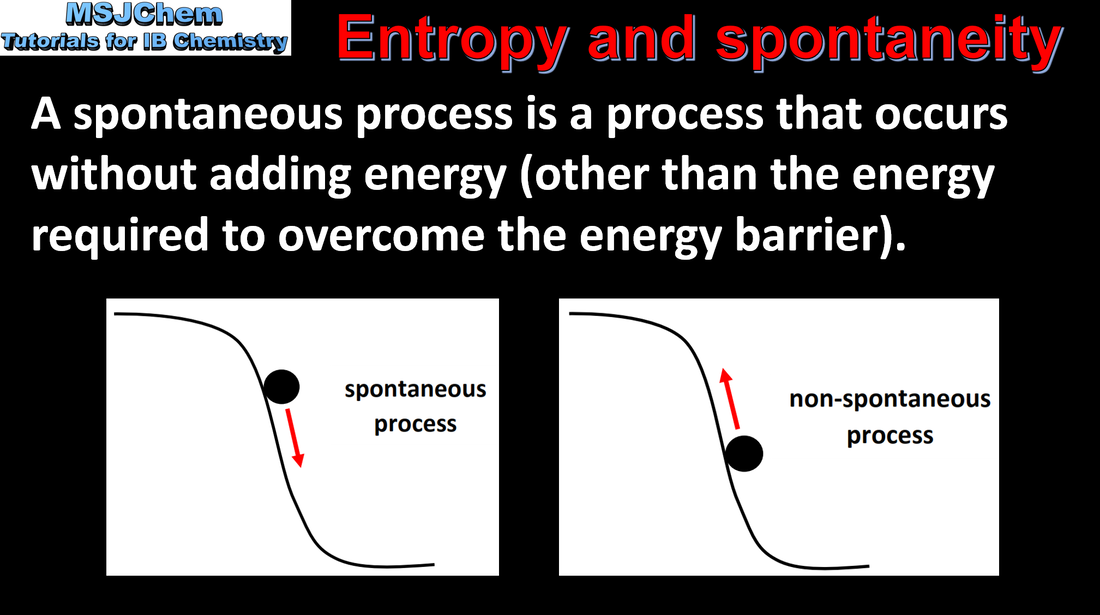
15.2 Entropy and spontaneity
|
Essential idea: A reaction is spontaneous if the overall transformation leads to an increase in total entropy (system plus surroundings). The direction of spontaneous change always increases the total entropy of the universe at the expense of energy available to do useful work. This is known as the second law of thermodynamics.
|
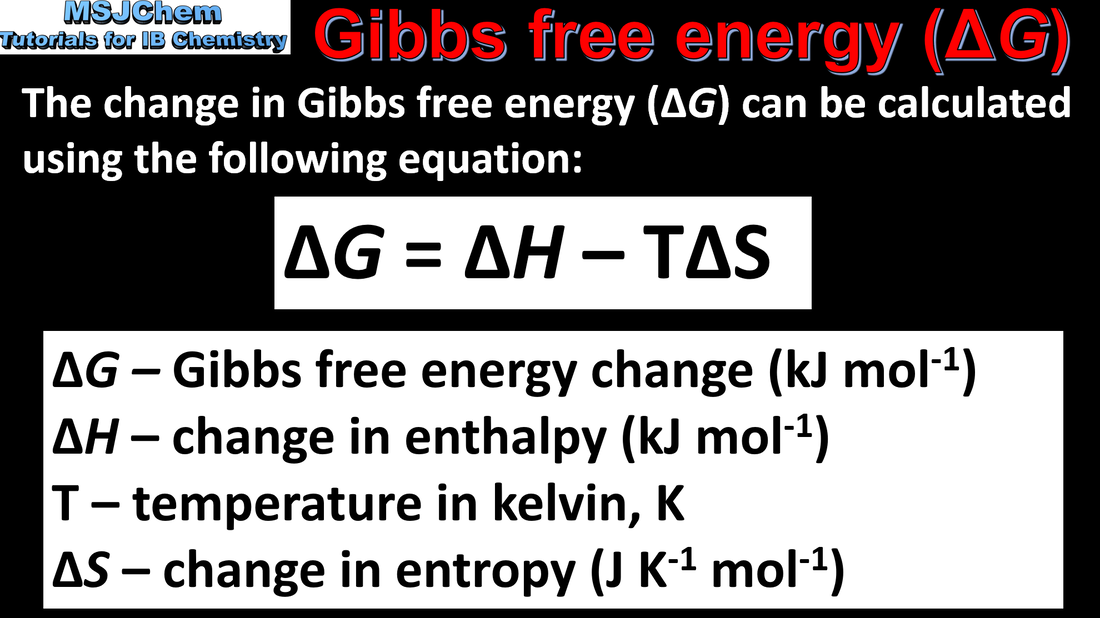
15.2 Gibbs free energy
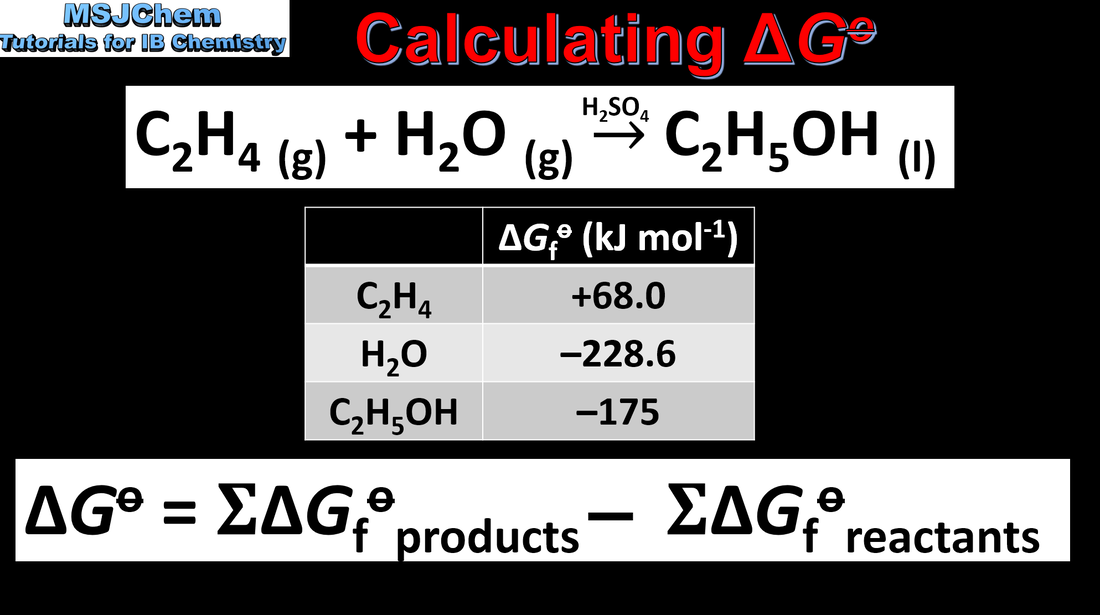
15.2 Calculating ∆G° using ∆G°f values
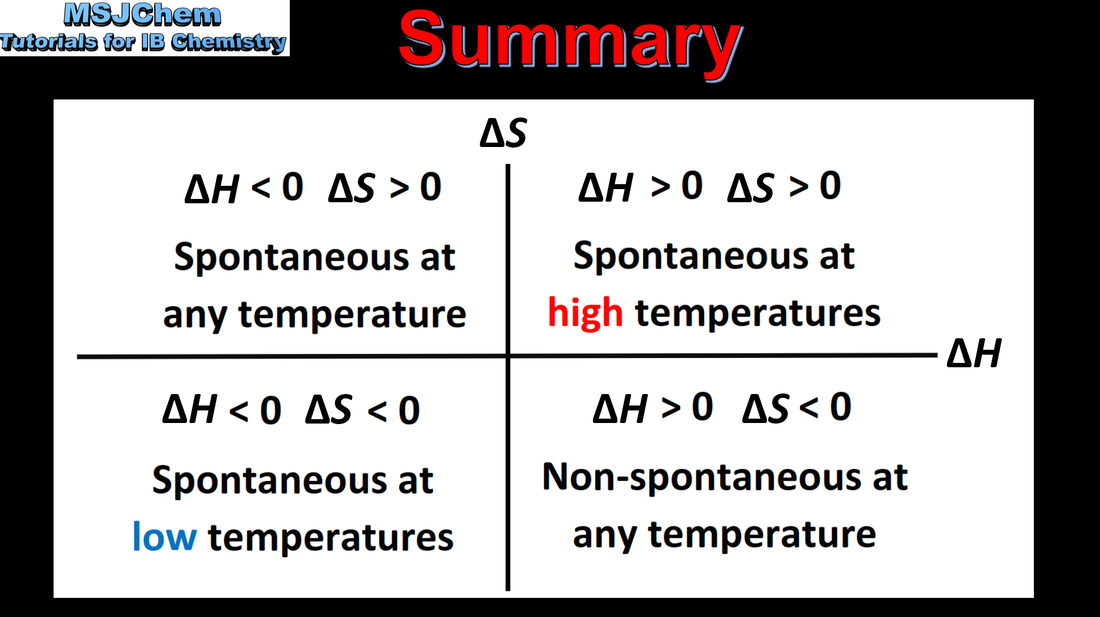
15.2 Effect of ΔH, ΔS and T on the spontaneity of a reaction
15.2 /17.1 ΔG and equilibrium
|
Understandings:
The position of equilibrium corresponds to a maximum value of entropy and a minimum in the value of the Gibbs free energy. The Gibbs free energy change of a reaction and the equilibrium constant can both be used to measure the position of an equilibrium reaction and are related by the equation, ∆G = −RT lnk Applications and skills: Relationship between ∆G and the equilibrium constant. Calculations using the equation ∆G = −RT lnk |