Structure 2.2 The covalent model
Structure 2.2.1 and 2.2.2
Understandings:
Understandings:
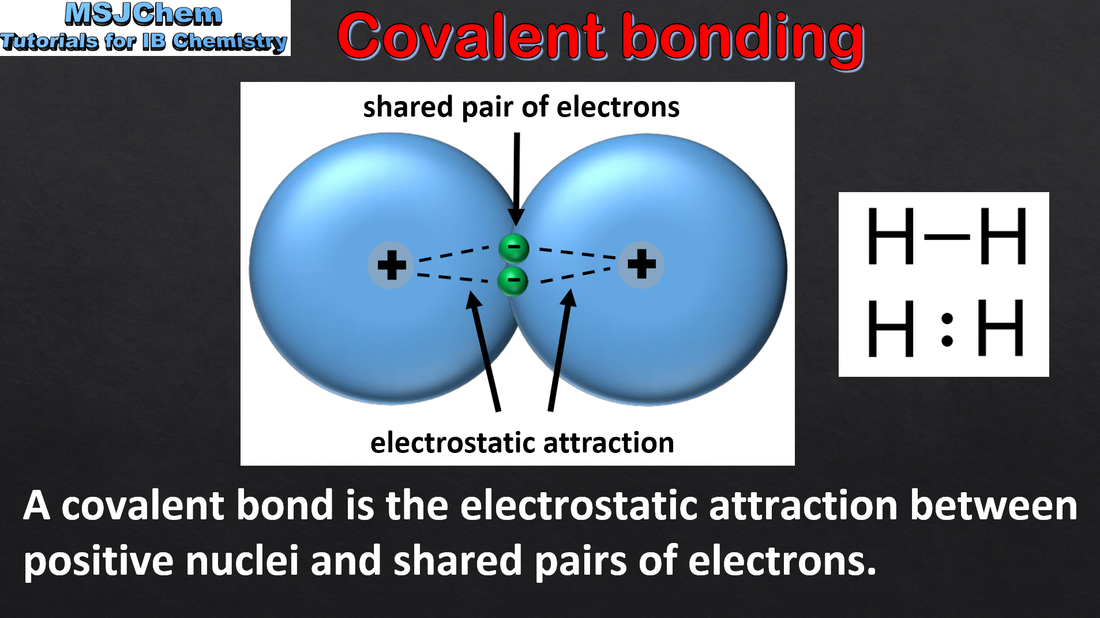
- A covalent bond is formed by the electrostatic attraction between a shared pair of electrons and the positively charged nuclei (2.2.1).
- The octet rule refers to the tendency of atoms to gain a valence shell with a total of 8 electrons (2.2.1).
- Single, double and triple bonds involve one, two and three shared pairs of electrons respectively (2.2.2).
- Deduce the Lewis formula of molecules and ions for up to four electron pairs on each atom (2.2.1).
- Explain the relationship between the number of bonds, bond length and bond strength (2.2.2).
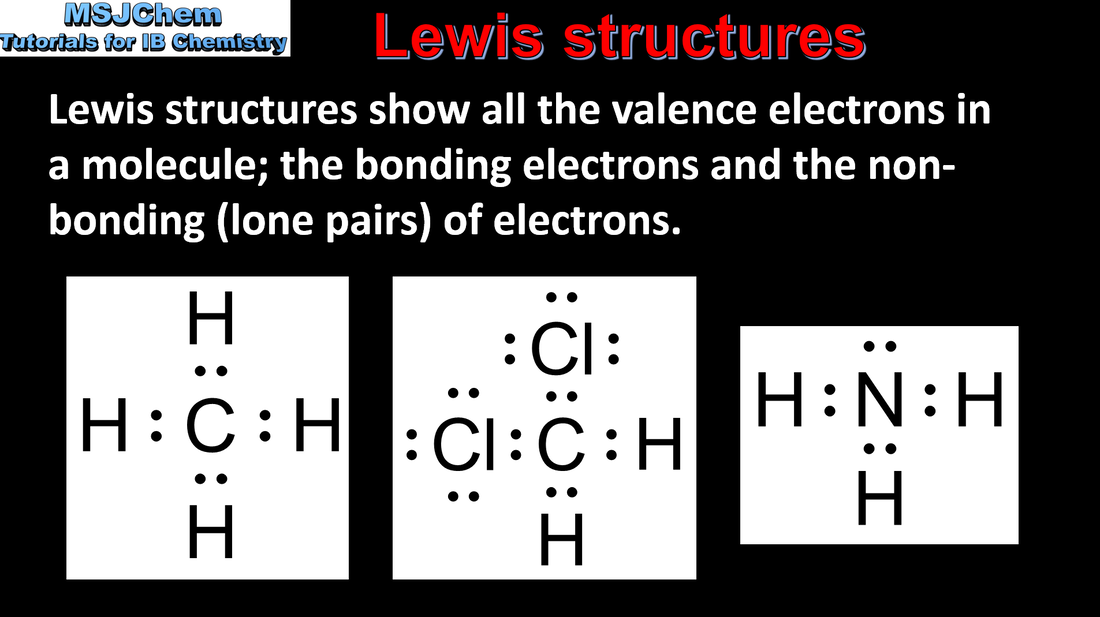
- Lewis formulas (also known as electron dot or Lewis structures) show all the valence electrons (bonding and non-bonding pairs) in a covalently bonded species.
- Electron pairs in a Lewis formula can be shown as dots, crosses or dashes.
- Molecules containing atoms with fewer than an octet of electrons should be covered.
- Organic and inorganic examples should be used.
- Structure 1.3 Why do noble gases form covalent bonds less readily than other elements?
- Structure 2.1 Why do ionic bonds only form between different elements while covalent bonds can form between atoms of the same element?
- Reactivity 2.2 How does the presence of double and triple bonds in molecules influence their reactivity?
Structure 2.2.3
Understandings:
Understandings:
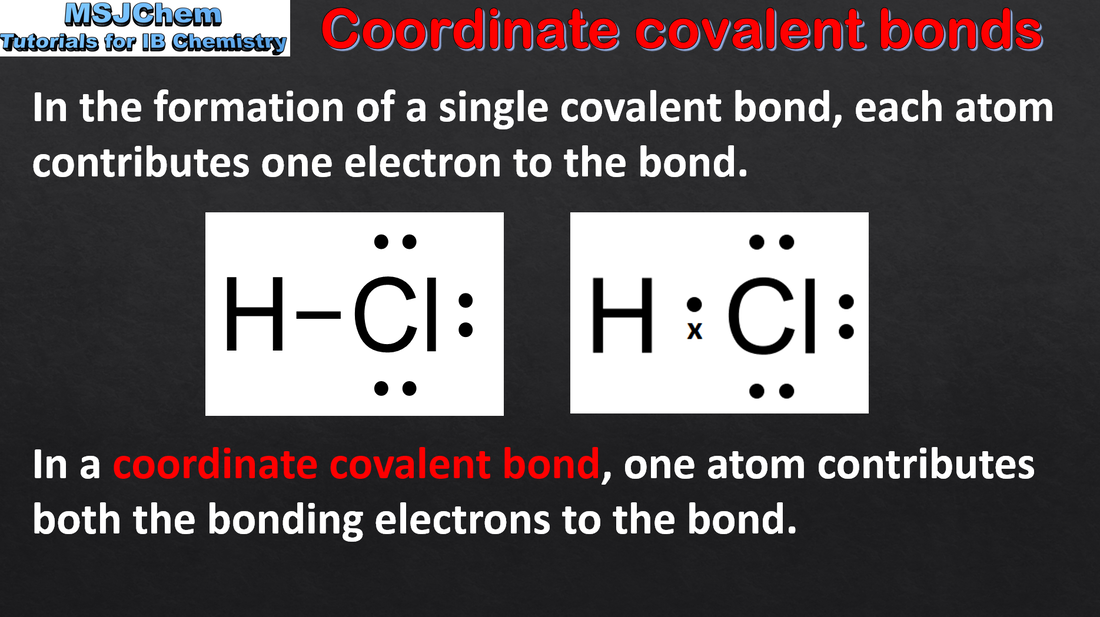
- A coordination bond is a covalent bond in which both the electrons of the shared pair originate from the same atom.
- Identify coordination bonds in compounds.
- Include coverage of transition element complexes (HL).
- Reactivity 3.4 (HL) Why do Lewis acid–base reactions lead to the formation of coordination bonds?
|
This video covers coordinate covalent bonds (note that the IB now refers to these type of bonds as coordination bonds according to the IUPAC definition).
|
Structure 2.2.4
Understandings:
Understandings:
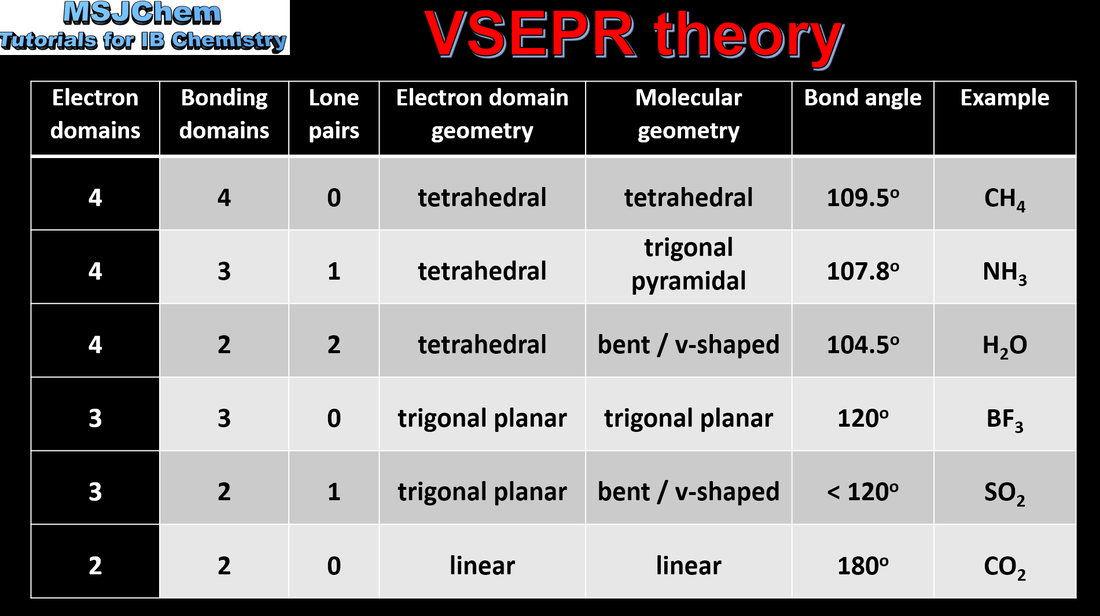
- The valence shell electron pair repulsion (VSEPR) model enables the shapes of molecules to be predicted from the repulsion of electron domains around a central atom.
- Predict the electron domain geometry and the molecular geometry for species with up to four electron domains.
- Include predicting how non-bonding pairs and multiple bonds affect bond angles.
Structure 2.2.5
Understandings:
Understandings:
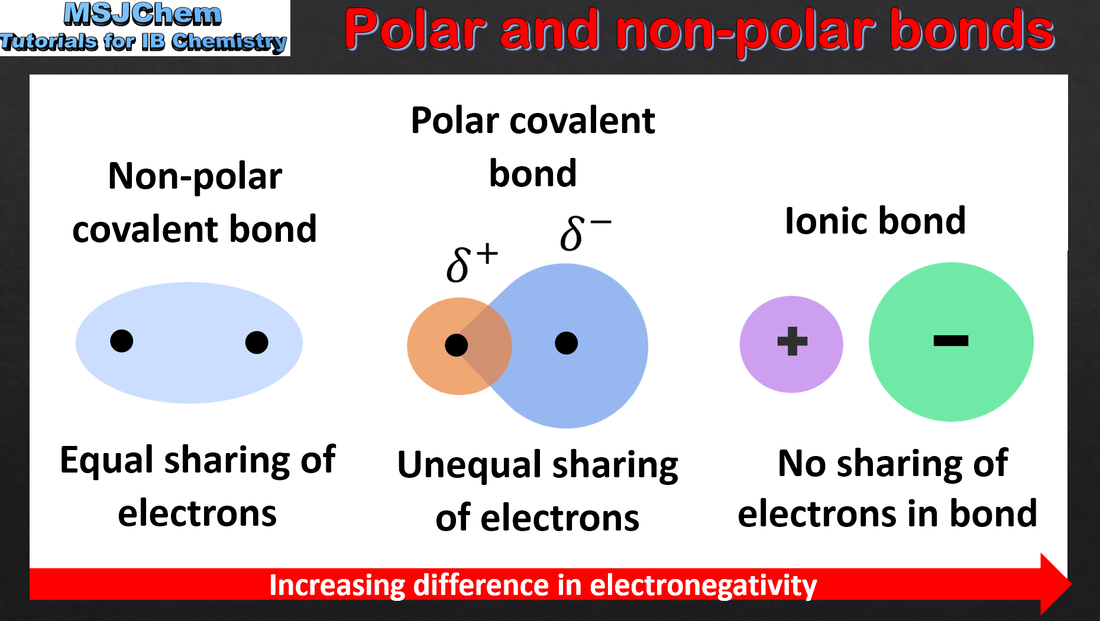
- Bond polarity results from the difference in electronegativities of the bonded atoms.
- Deduce the polar nature of a covalent bond from electronegativity values.
- Bond dipoles can be shown either with partial charges or vectors.
- Electronegativity values are given in the data booklet.
- Structure 2.1 What properties of ionic compounds might be expected in compounds with polar covalent bonding?
Structure 2.2.6
Understandings:
Understandings:
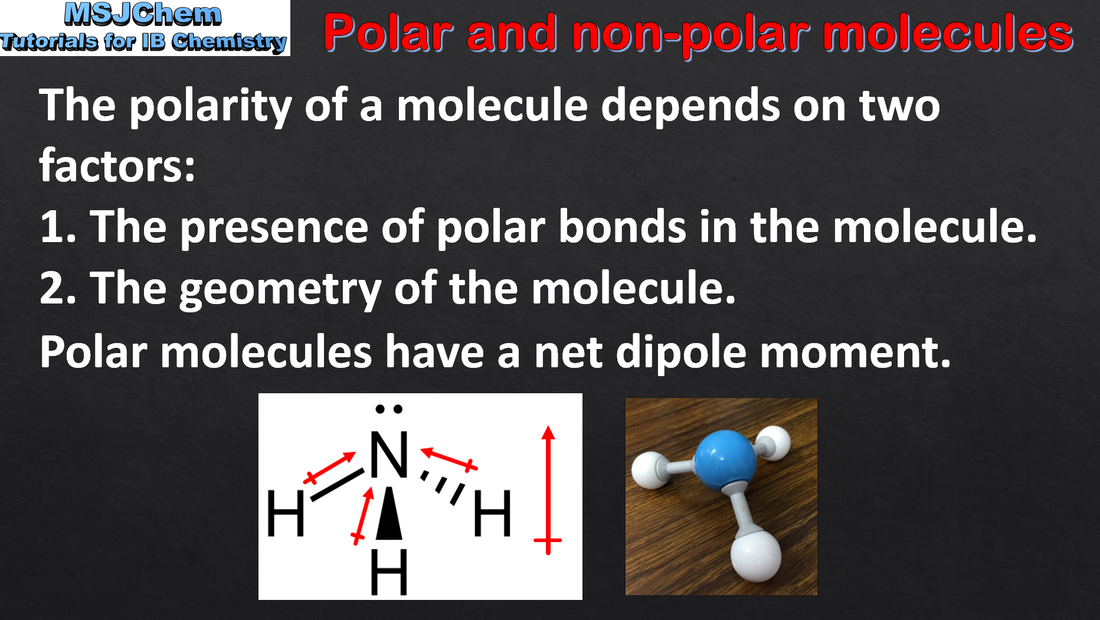
- Molecular polarity depends on both bond polarity and molecular geometry.
- Deduce the net dipole of a molecule or ion by considering bond polarity and geometry.
- Examples should include species in which bond dipoles do and do not cancel each other.
- Structure 3.2 (HL) What features of a molecule make it “infrared (IR) active”?
Structure 2.2.7
Understandings:
Understandings:
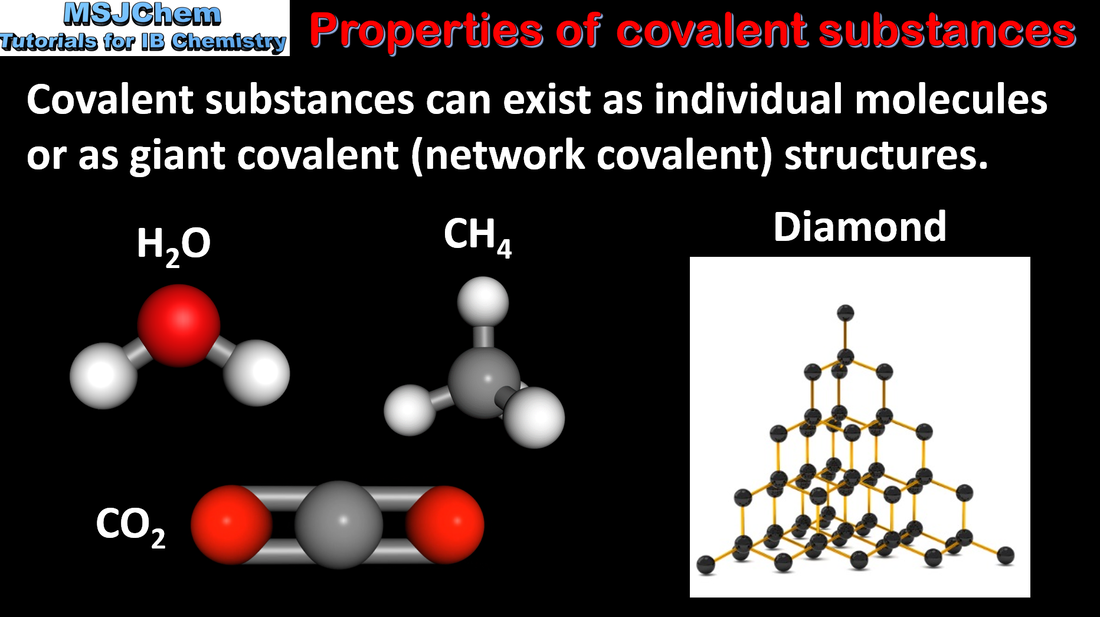
- Carbon and silicon form covalent network structures.
- Describe the structures and explanation of the properties of silicon, silicon dioxide and carbon’s allotropes: diamond, graphite, fullerenes and graphene.
- Allotropes of the same element have different bonding and structural patterns, and so have different chemical and physical properties.
- Structure 3.1 Why are silicon–silicon bonds generally weaker than carbon–carbon bonds?
Structure 2.2.8 and 2.2.9
Understandings:
Understandings:
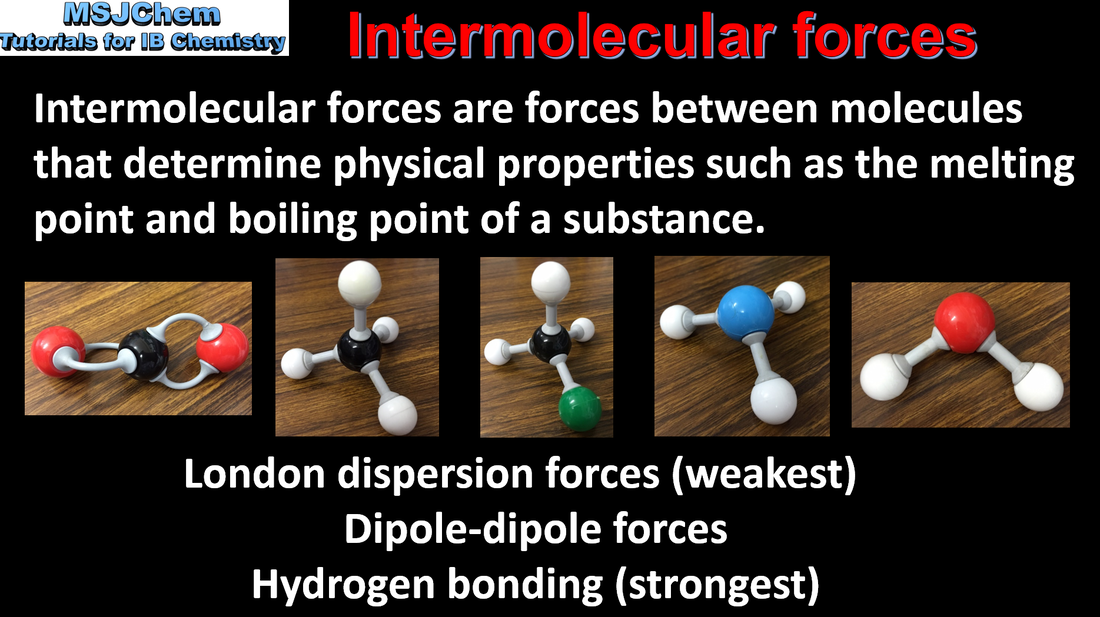
- The nature of the force that exists between molecules is determined by the size and polarity of the molecules.
- Intermolecular forces include London (dispersion), dipole-induced dipole, dipole–dipole and hydrogen bonding.
- Given comparable molar mass, the relative strengths of intermolecular forces are generally: London (dispersion) forces < dipole–dipole forces < hydrogen bonding.
- Deduce the types of intermolecular force present from the structural features of covalent molecules.
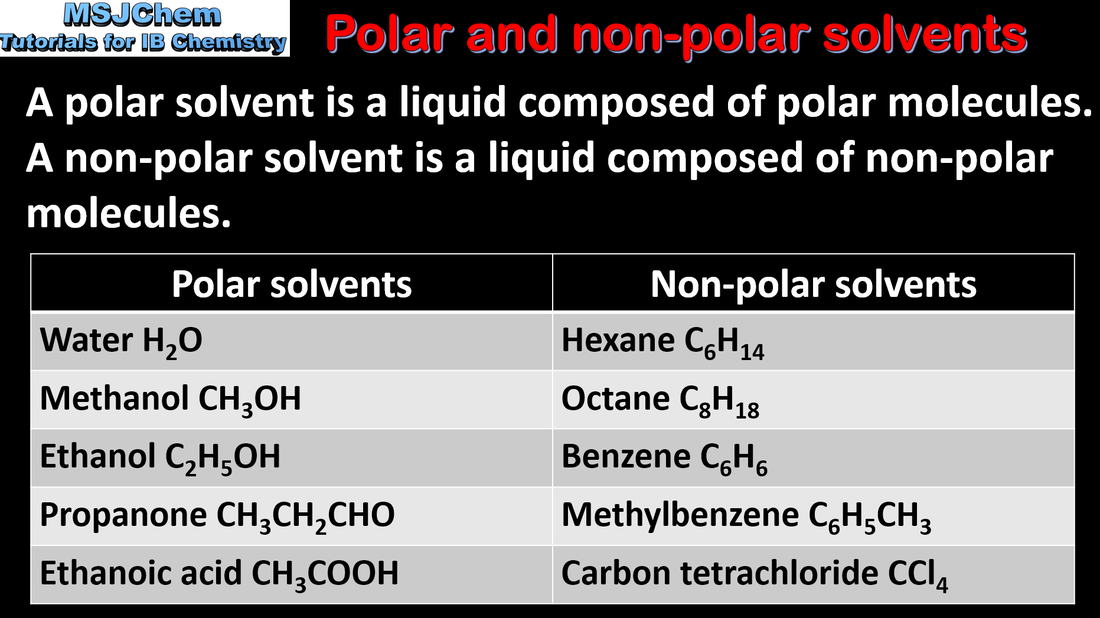
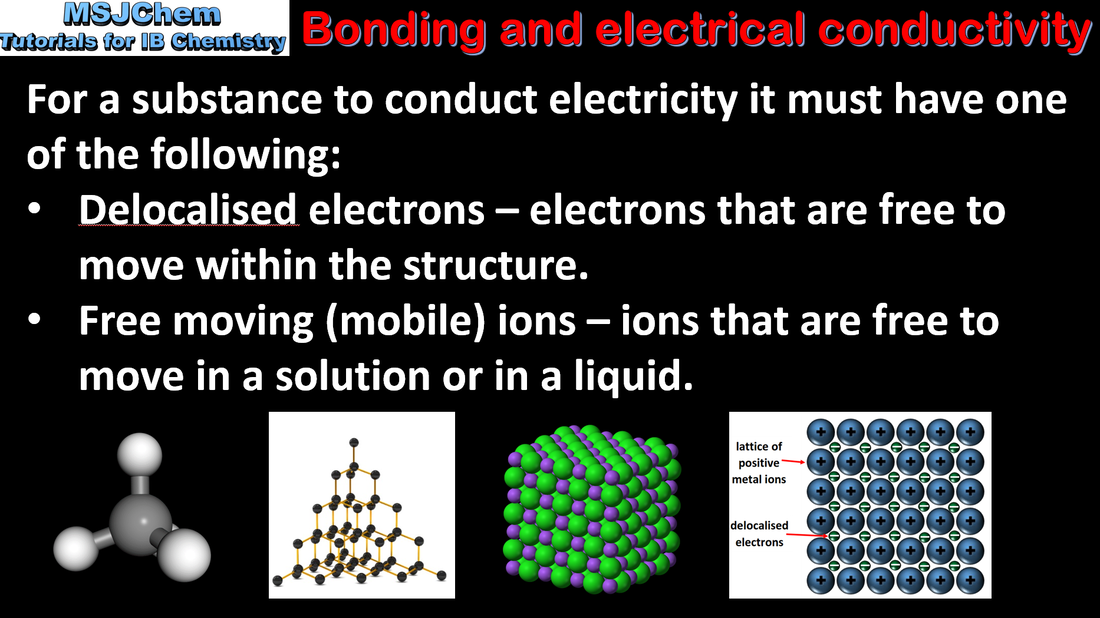
- Explain the physical properties of covalent substances to include volatility, electrical conductivity and solubility in terms of their structure.
- The term “van der Waals forces” should be used as an inclusive term to include dipole–dipole, dipole- induced dipole, and London (dispersion) forces.
- Hydrogen bonds occur when hydrogen, being covalently bonded to an electronegative atom, has an attractive interaction on a neighbouring electronegative atom.
- Structure 1.5 To what extent can intermolecular forces explain the deviation of real gases from ideal behaviour?
- Structure 3.2 To what extent does a functional group determine the nature of the intermolecular forces?
Structure 2.2.10
Understandings:
Understandings:
- Chromatography is a technique used to separate the components of a mixture based on their relative attractions involving intermolecular forces to mobile and stationary phases.
- Explain, calculate and interpret the retardation factor values, RF.
- The use of locating agents is not required.
- The operational details of a gas chromatograph or high-performance liquid chromatograph will not be assessed.