Reactivity 1.2 Energy cycles in reactions (HL)
Reactivity 1.2.3 and 1.2.4
Understandings:
Understandings:
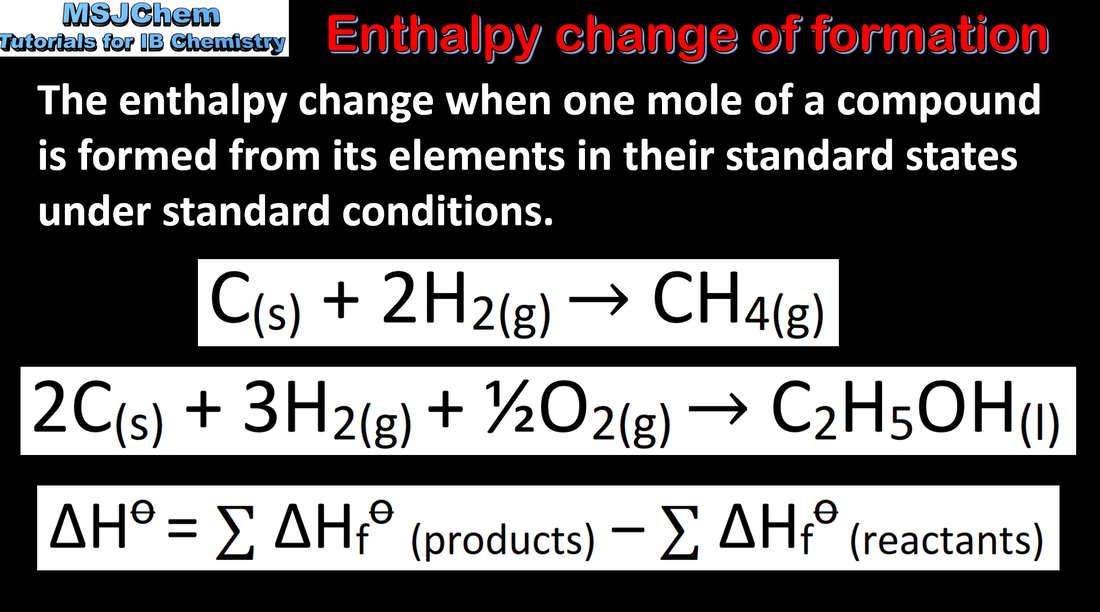
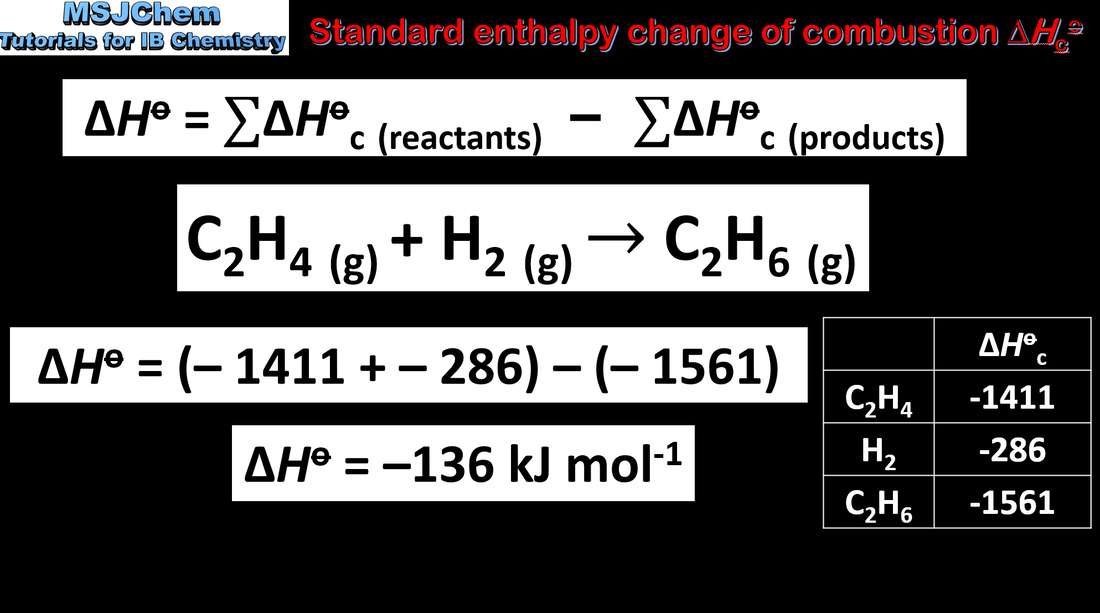
- Standard enthalpy changes of combustion, ΔHc, and enthalpy of formation, ΔHf, data are used in thermodynamic calculations (1.2.3).
- An application of Hess’s law uses enthalpy of formation data or enthalpy of combustion data to calculate the enthalpy change (1.2.4).
- Deduce equations and solutions to problems involving these terms (1.2.3).
- Calculate enthalpy changes of a reaction using ΔHf data or ΔHc data (1.2.4).
- ΔH = ΣΔHc reactants − ΣΔHc products
- ΔH = ΣΔHf products − ΣΔHf reactants
- Enthalpy of combustion and formation data are given in the data booklet.
- The above equations are given in the data booklet.
- Structure 2.2 Would you expect allotropes of an element, such as diamond and graphite, to have different ΔH values?
Reactivity 1.2.5
Understandings:
Understandings:
- A Born–Haber cycle is an application of Hess’s law, used to show energy changes in the formation of an ionic compound.
- Interpret and determine values from a Born–Haber cycle for compounds composed of univalent and divalent ions.
- The cycle includes: ionization energies, enthalpy of atomization (using sublimation and/or bond enthalpies), electron affinities, lattice enthalpy, enthalpy of formation.
- The construction of a complete Born–Haber cycle will not be assessed.
- Structure 2.1 What are the factors that influence the strength of lattice enthalpy in an ionic compound?
|
This video covers using a Born Haber cycle to calculate the enthalpy of formation of sodium chloride.
|