Topic 20 Organic chemistry HL
20.1 Organic acid and bases
|
|
This video covers organic acids and bases.
|
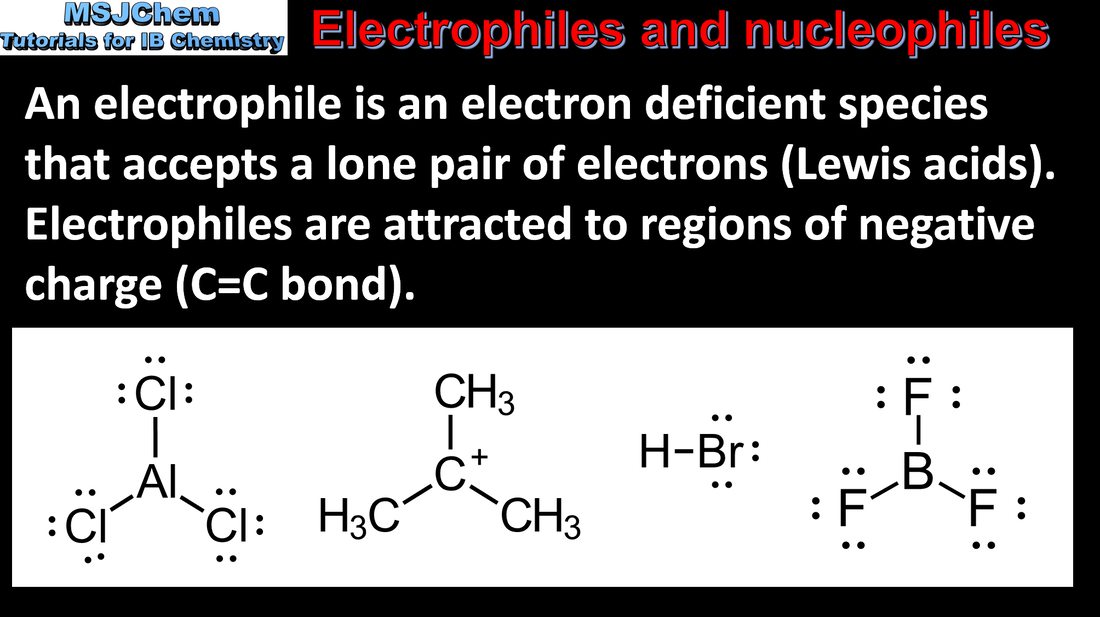
20.1 Electrophiles and nucleophiles
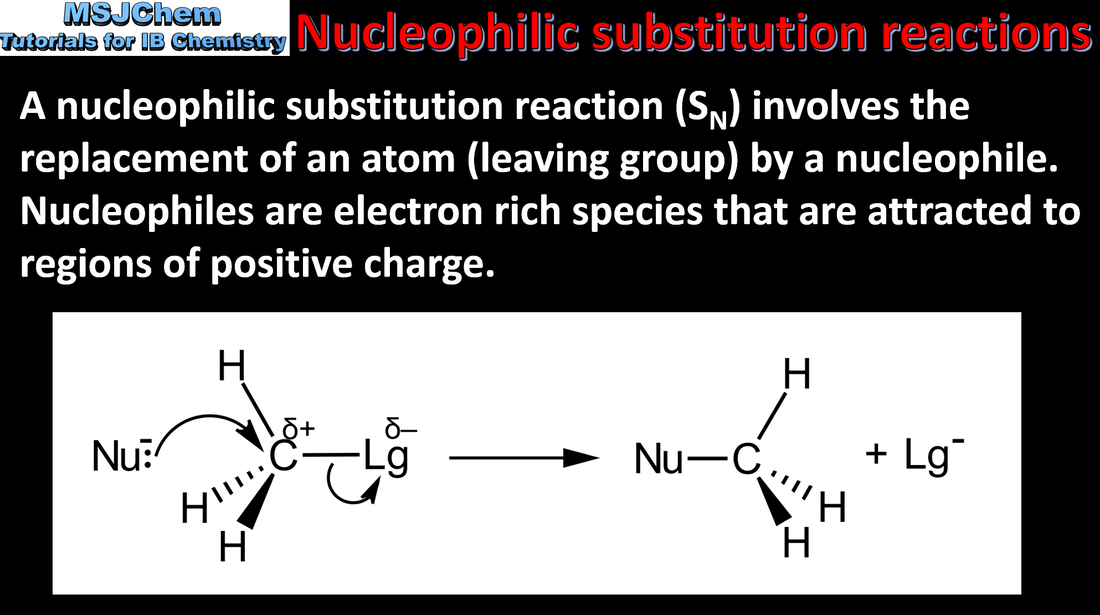
20.1 Introduction to nucleophilic substitution reactions
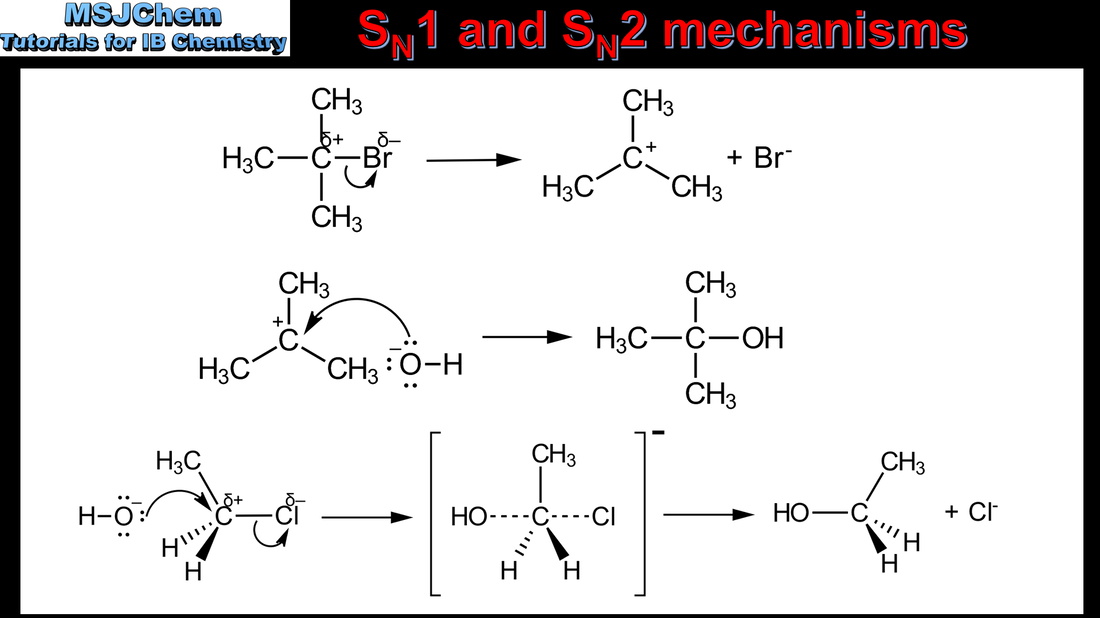
20.1 SN1 and SN2 mechanisms
|
Understandings:
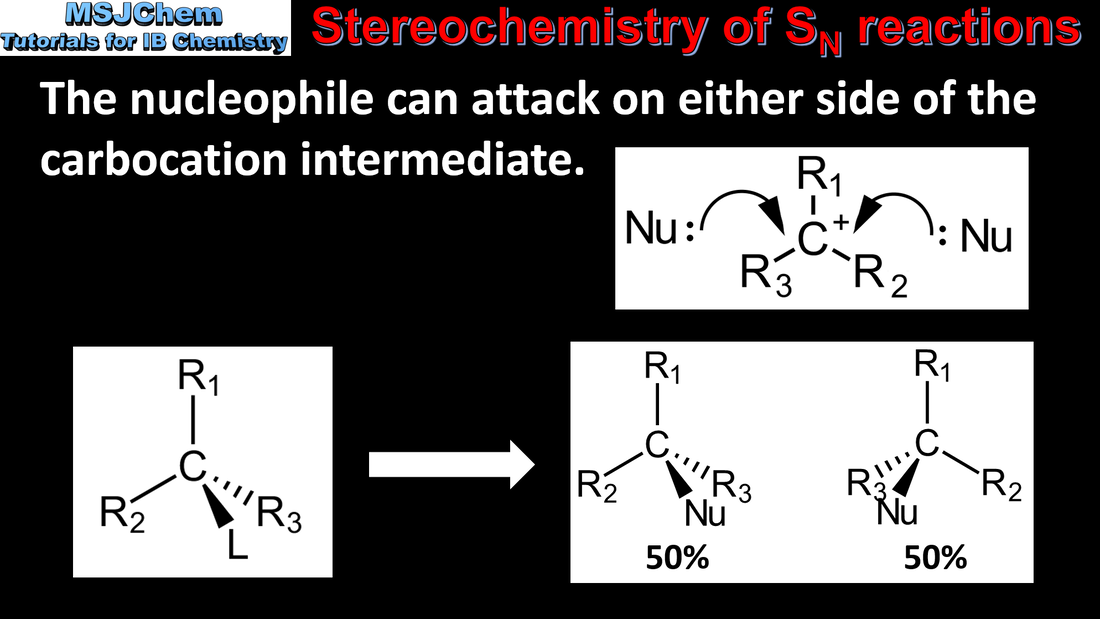
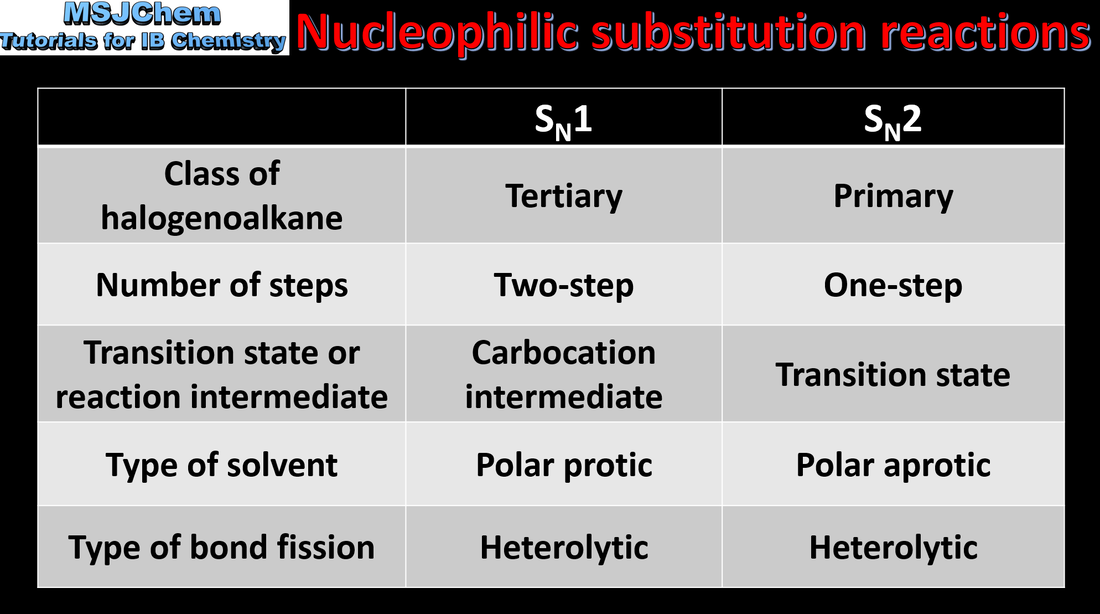
SN1 represents a nucleophilic unimolecular substitution reaction. SN1 involves a carbocation intermediate. For tertiary halogenoalkanes the predominant mechanism is SN1 The rate determining step (slow step) in an SN1 reaction depends only on the concentration of the halogenoalkane, rate = k[halogenoalkane]. SN2 represents a nucleophilic bimolecular substitution reaction. SN2 involves a concerted reaction with a transition state. For primary halogenoalkanes the predominant mechanism is SN2. Both mechanisms occur for secondary halogenoalkanes. For SN2, rate = k[halogenoalkane][nucleophile]. SN2 is stereospecific with an inversion of configuration at the carbon. SN2 reactions are best conducted using aprotic, polar solvents. Applications and skills: Deduction of the mechanism of the nucleophilic substitution reactions of halogenoalkanes with aqueous sodium hydroxide in terms of SN1 and SN2 mechanisms. |
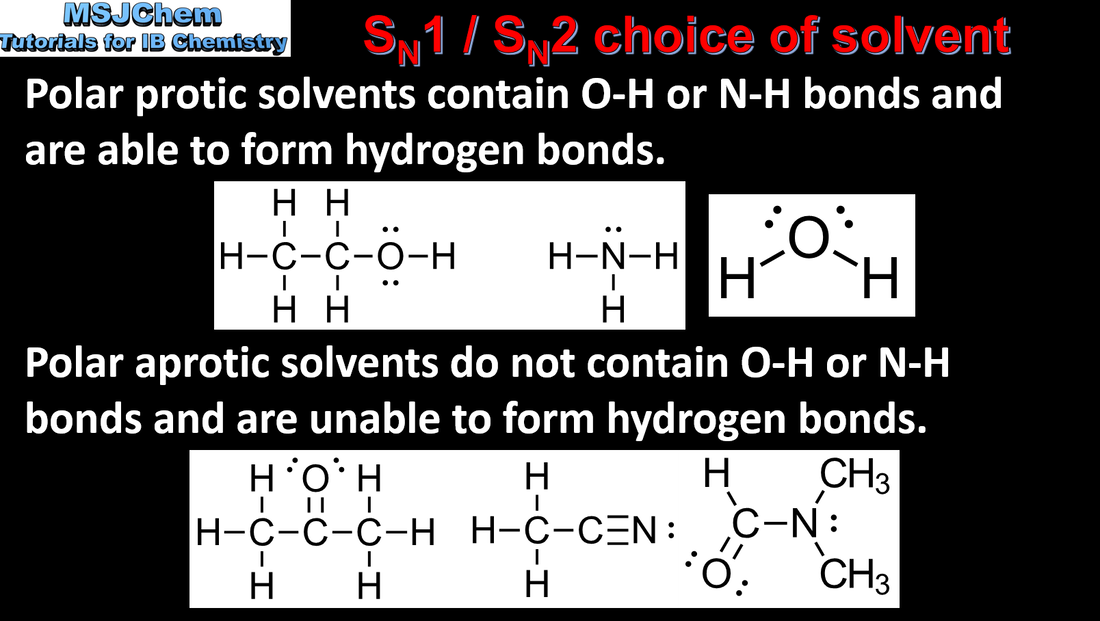
20.1 Choice of solvent for SN1 and SN2 reactions
20.1 Stereochemistry of SN reactions
20.1 Comparison of SN1 and SN2 reactions
20.1 Comparison on hydroxide ion and water molecule as nucleophiles
|
|
Applications and skills:
Explanation of why hydroxide is a better nucleophile than water. |
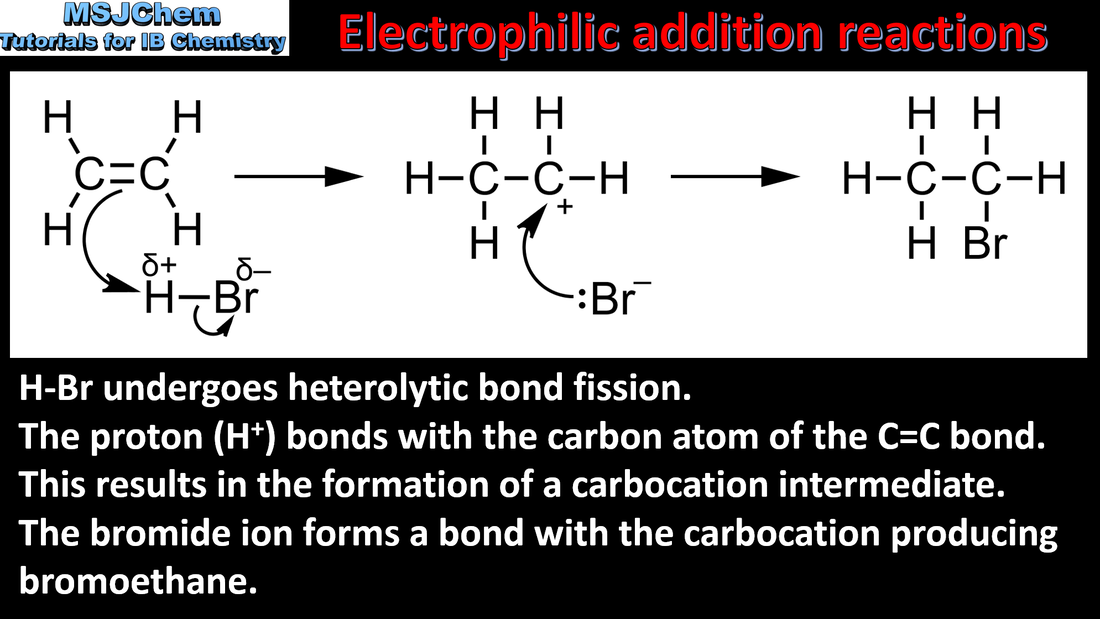
20.1 Electrophilic addition reactions
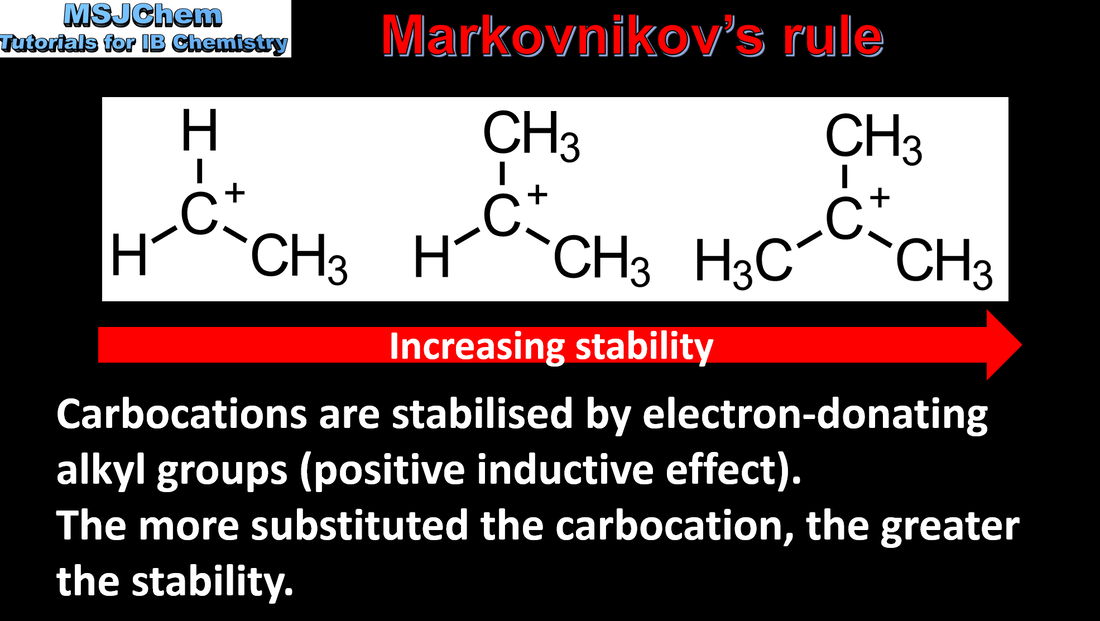
20.1 Markovnikov's rule
|
Understandings:
Markovnikov’s rule can be applied to predict the major product in electrophilic addition reactions of unsymmetrical alkenes with hydrogen halides and interhalogens. The formation of the major product can be explained in terms of the relative stability of possible carbocations in the reaction mechanism. |
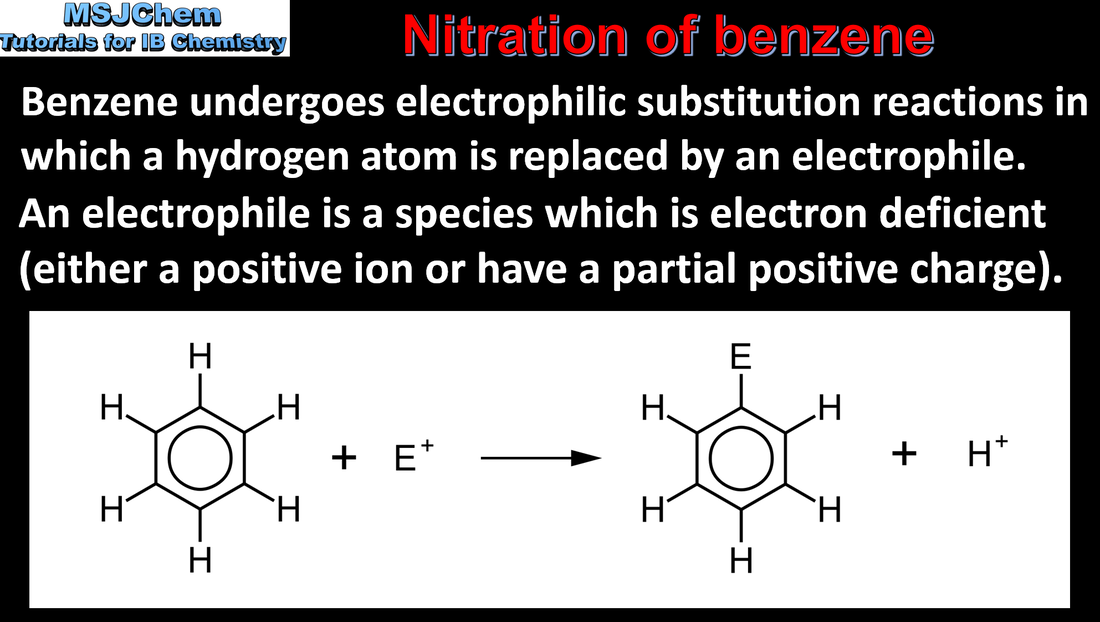
20.1 Nitration of benzene
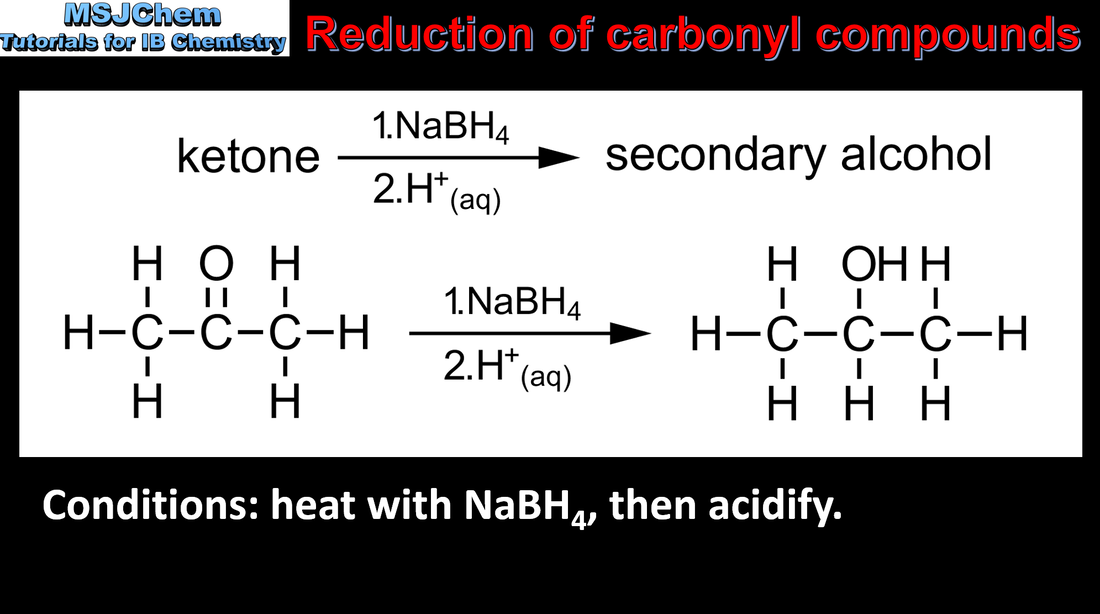
20.1 Reduction of carbonyl compounds
|
I should point out that the reduction of a carboxylic acid using lithium aluminium hydride first involves the formation of an aldehyde which then immediately reacts with the reducing agent to form a primary alcohol. So basically it is not possible to obtain an aldehyde using lithium aluminium hydride (although it is produced in the reaction). There are other ways to reduce a carboxylic acid to an aldehyde such as Fukuyama reduction, although this is not covered in the IB chemistry syllabus.
|
Understandings:
Reduction Reactions: Carboxylic acids can be reduced to primary alcohols (via the aldehyde). Ketones can be reduced to secondary alcohols. Typical reducing agents are lithium aluminium hydride (used to reduce carboxylic acids) and sodium borohydride. Applications and skills: Writing reduction reactions of carbonyl containing compounds: aldehydes and ketones to primary and secondary alcohols and carboxylic acids to primary alcohols, using suitable reducing agents. |
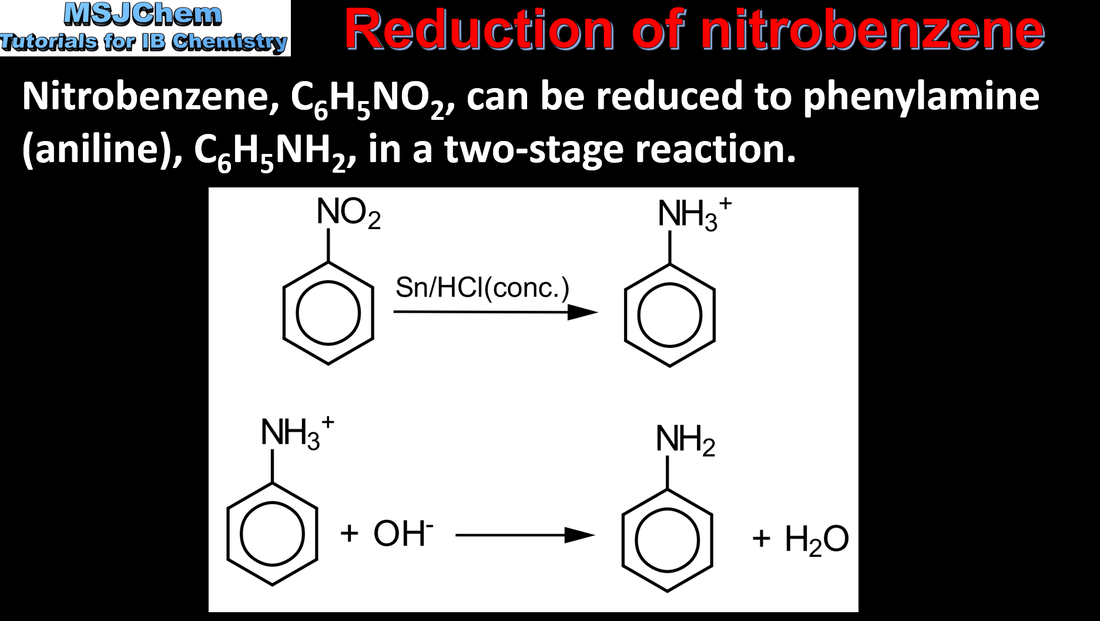
20.1 Reduction of nitrobenzene
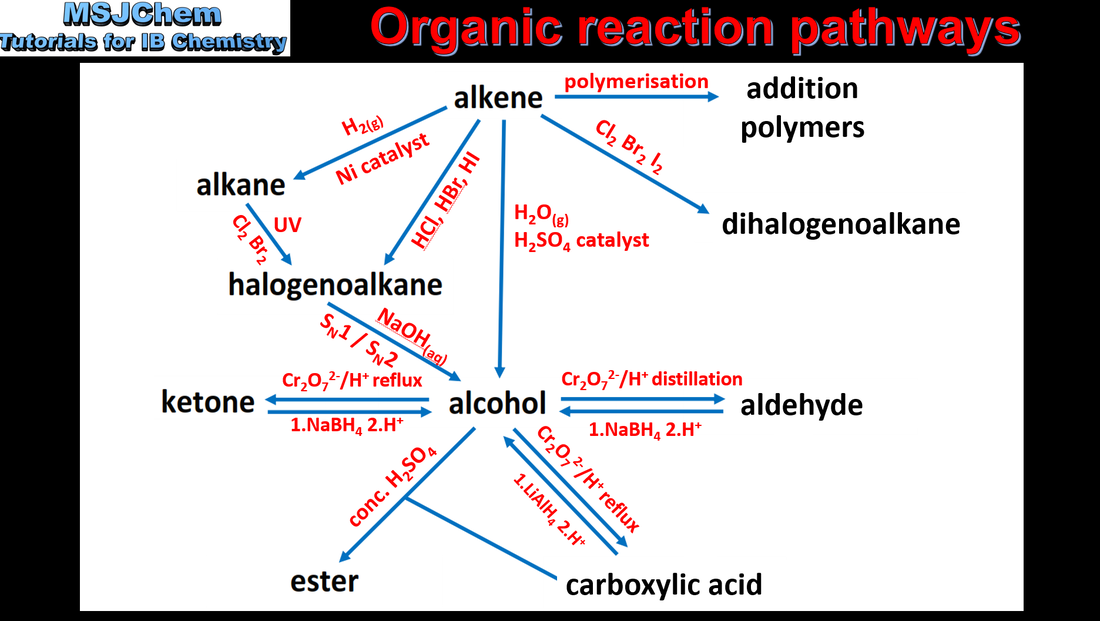
20.2 Organic reaction pathways
20.2 Retro-synthesis
|
|
Understandings:
Retro-synthesis of organic compounds. Applications and skills: Deduction of multi-step synthetic routes given starting reagents and the product(s). Guidance: Conversions with more than four stages will not be assessed in synthetic routes. Reaction types can cover any of the reactions covered in topic 10 and sub-topic 20.1. |
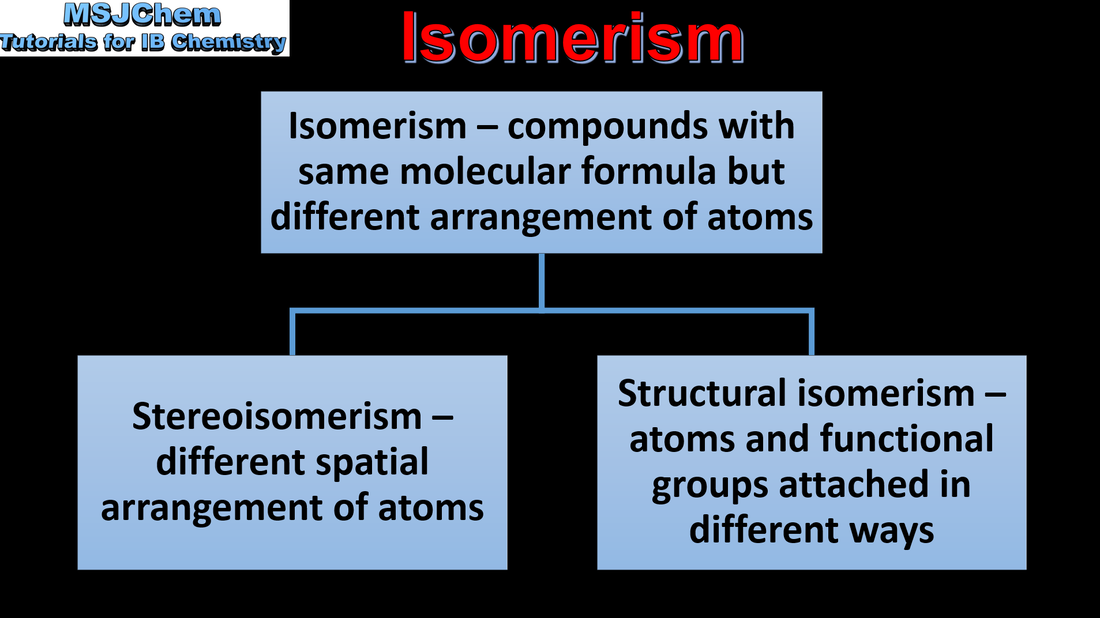
20.3 Introduction to isomerism
|
Understandings:
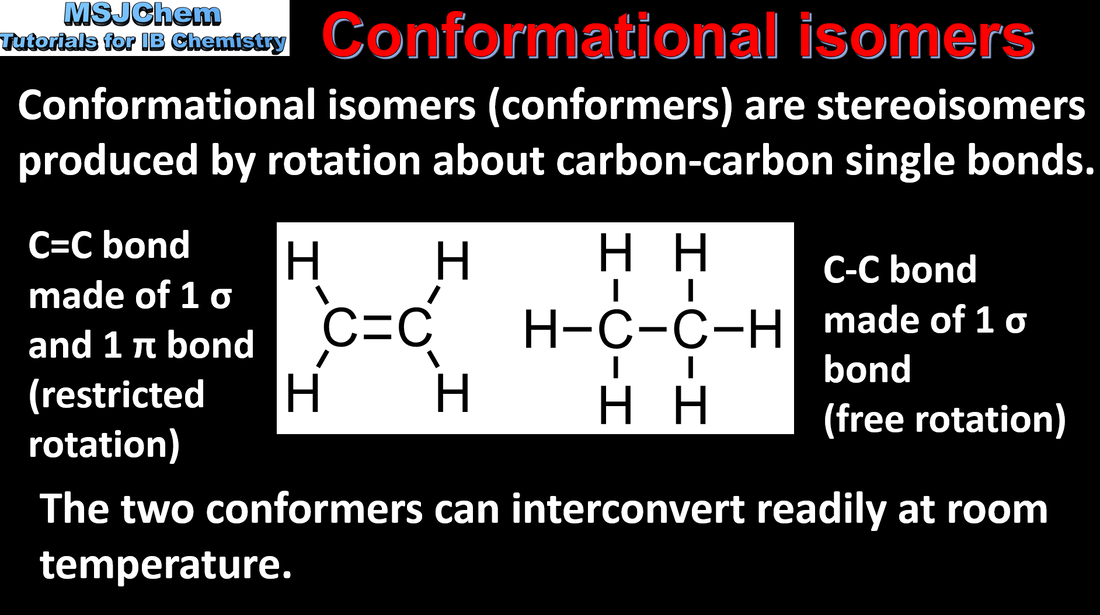
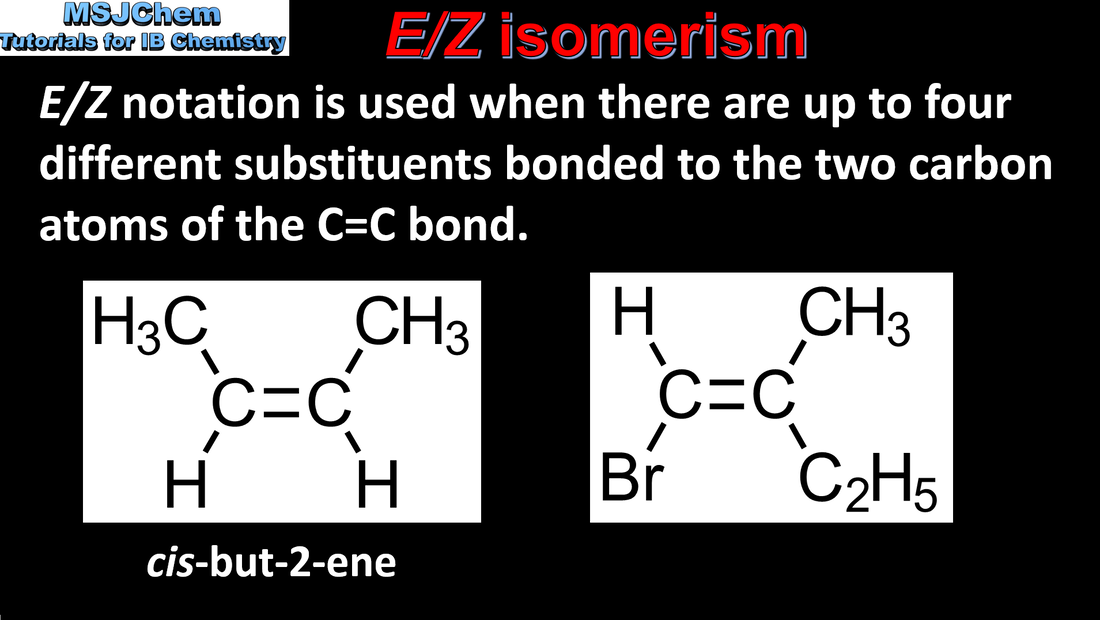
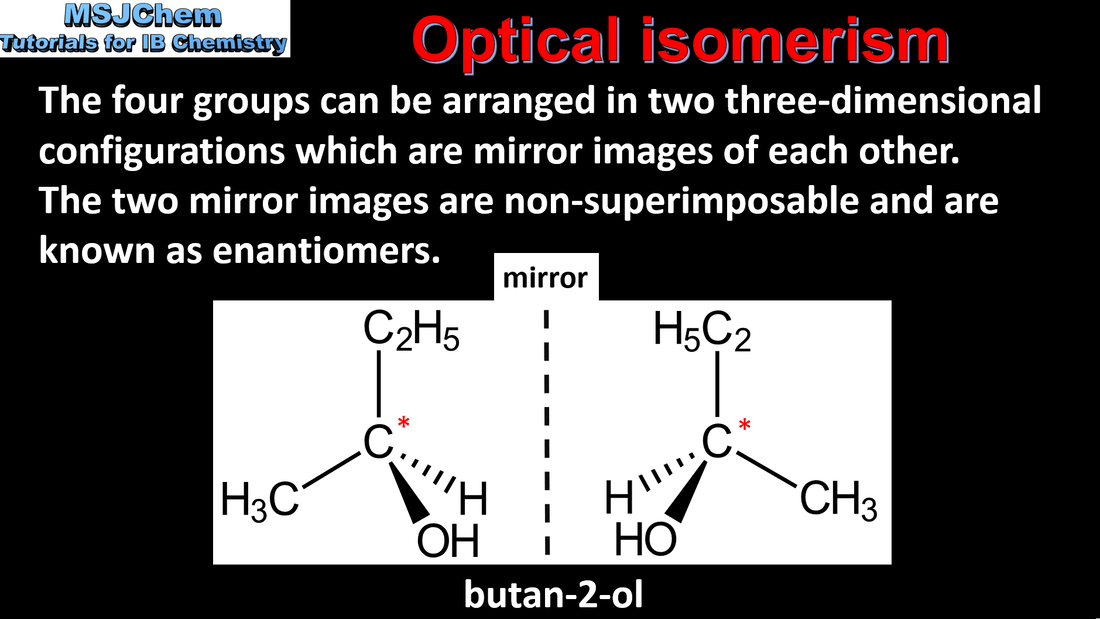
Stereoisomers are subdivided into two classes—conformational isomers, which interconvert by rotation about a σ bond and configurational isomers that interconvert only by breaking and reforming a bond. Configurational isomers are further subdivided into cis-trans and E/Z isomers and optical isomers. |
20.3 Conformational isomerism
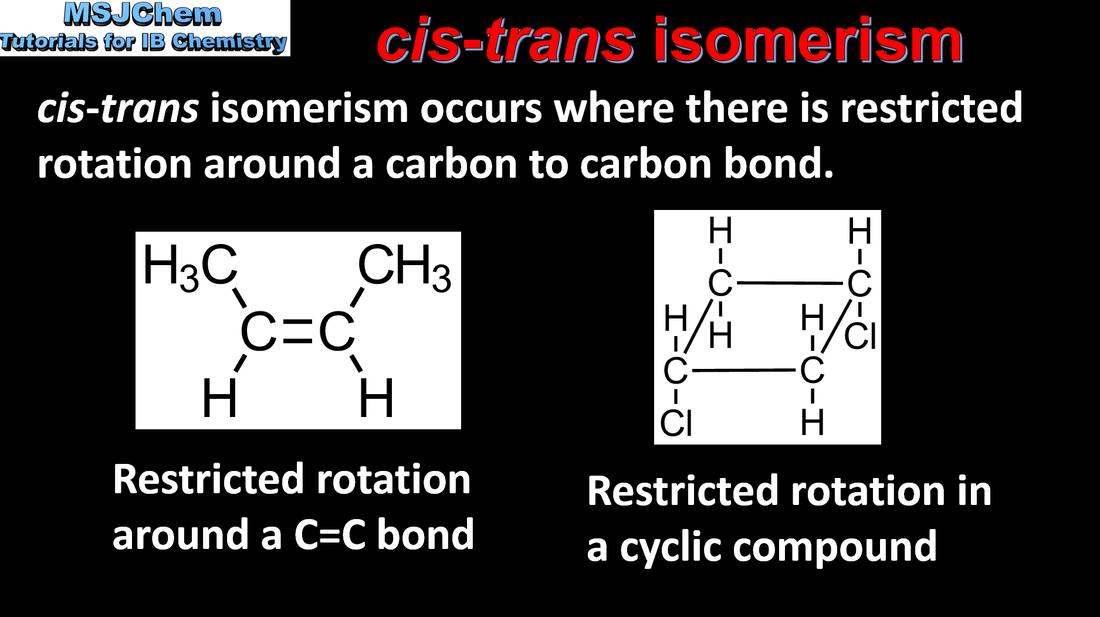
20.3 cis-trans isomerism
|
Understandings:
Cis-trans isomers can occur in alkenes or cycloalkanes (or heteroanalogues) and differ in the positions of atoms (or groups) relative to a reference plane. Applications and skills: Construction of 3-D models (real or virtual) of a wide range of stereoisomers. Guidance: The term geometric isomers as recommended by IUPAC is now obsolete and cis-trans isomers and E/Z isomers should be encouraged in the teaching programme. |
20.3 Physical properties of cis-trans isomers
|
|
This video covers the differences in melting point and boiling point between cis-trans isomers.
|
20.3 E/Z isomerism
20.3 Optical isomerism
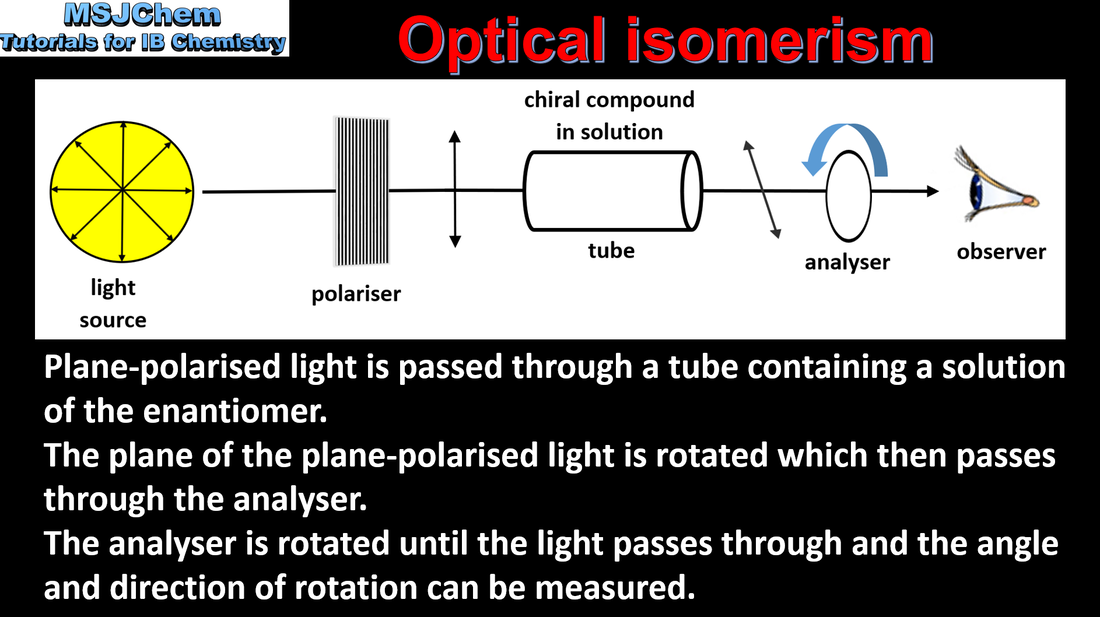
20.3 Optical isomerism - use of a polarimeter
|
Understandings:
An optically active compound can rotate the plane of polarized light as it passes through a solution of the compound. A racemic mixture (or racemate) is a mixture of two enantiomers in equal amounts and is optically inactive. Applications and skills: Distinction between optical isomers using a polarimeter. |
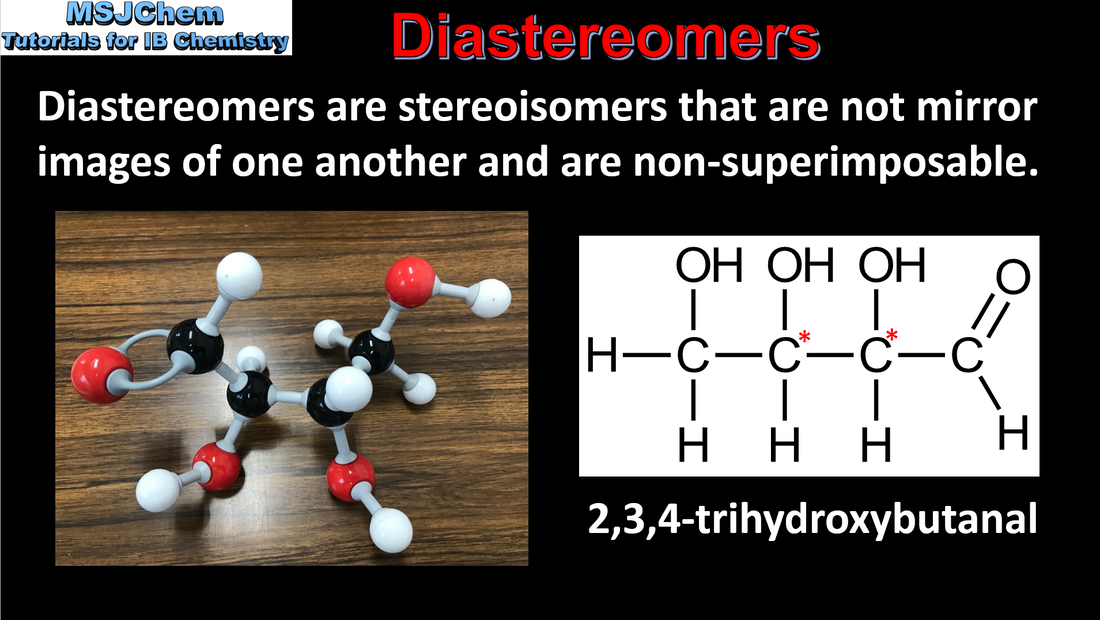
20.3 Diastereomers