Structure 3.1 The periodic table: Classification of elements
Structure 3.1.1 and 3.1.2
Understandings:
Understandings:
- The periodic table consists of periods, groups and blocks (3.1.1).
- The period number shows the outer energy level that is occupied by electrons. Elements in a group have a common number of valence electrons (3.1.2).
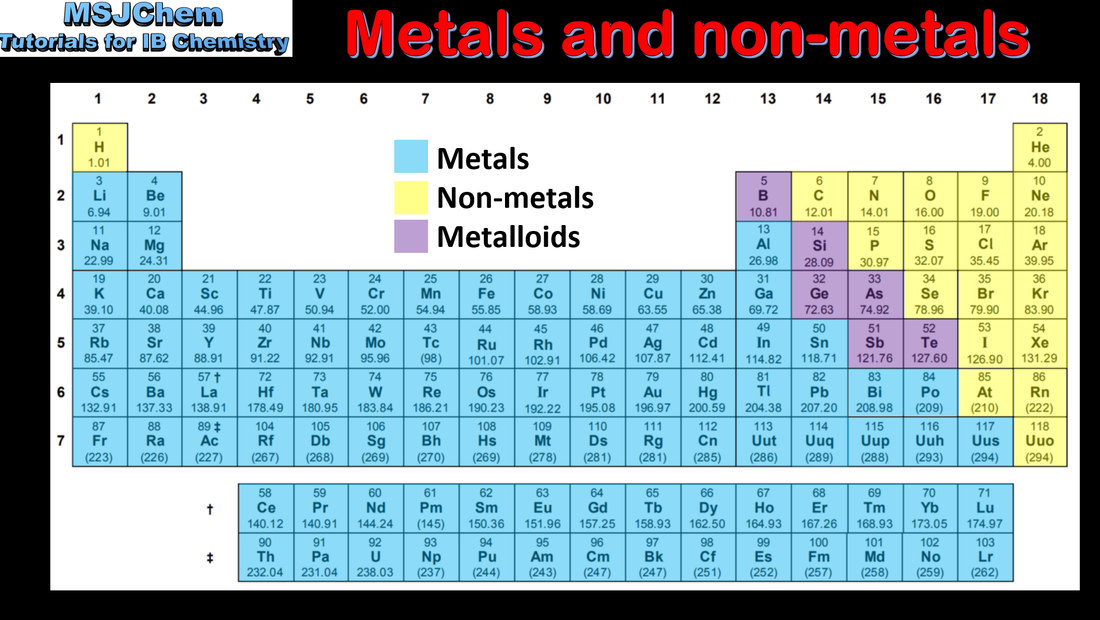
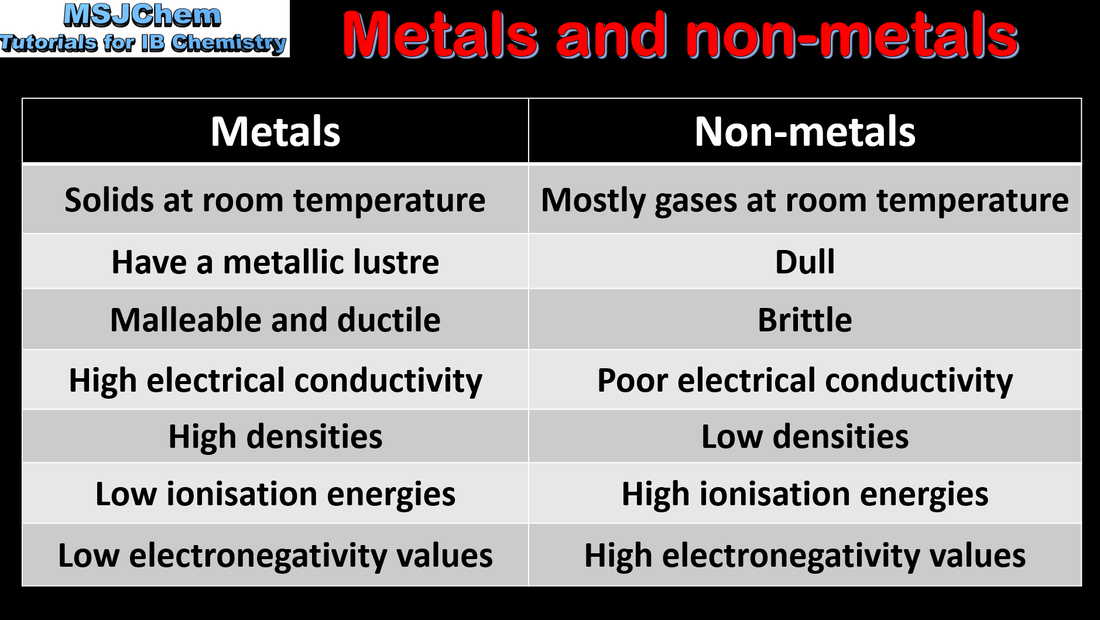
- Identify the positions of metals, metalloids and non-metals in the periodic table (3.1.1).
- Deduce the electron configuration of an atom up to Z = 36 from the element’s position in the periodic table and vice versa (3.1.2).
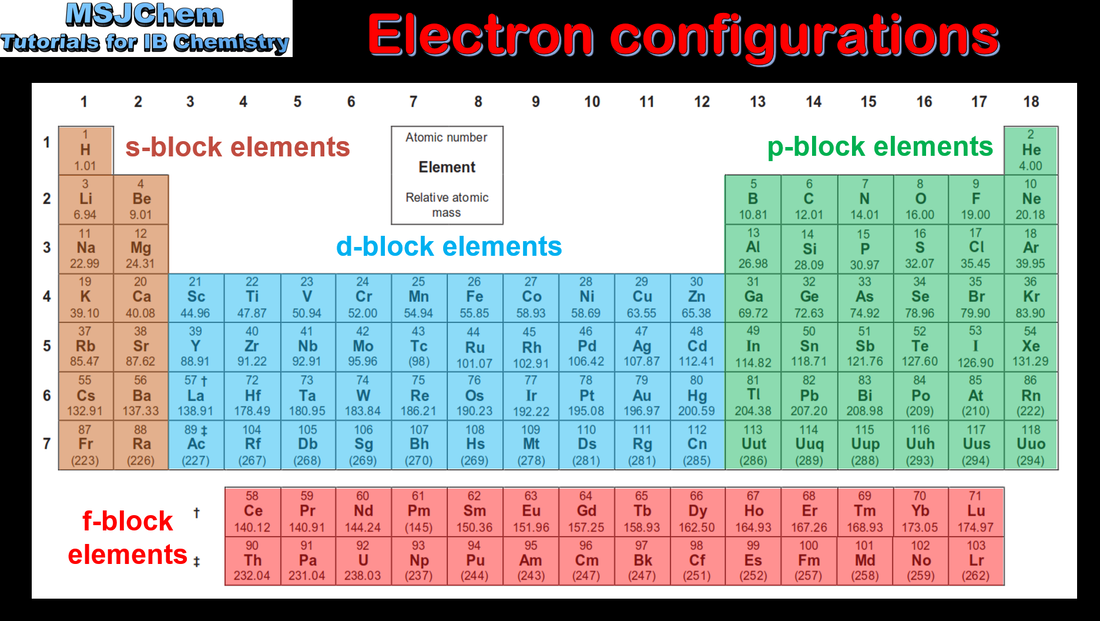
- The four blocks associated with the sublevels s, p, d, f should be recognized.
- A copy of the periodic table is available in the data booklet.
- Groups are numbered from 1 to 18.
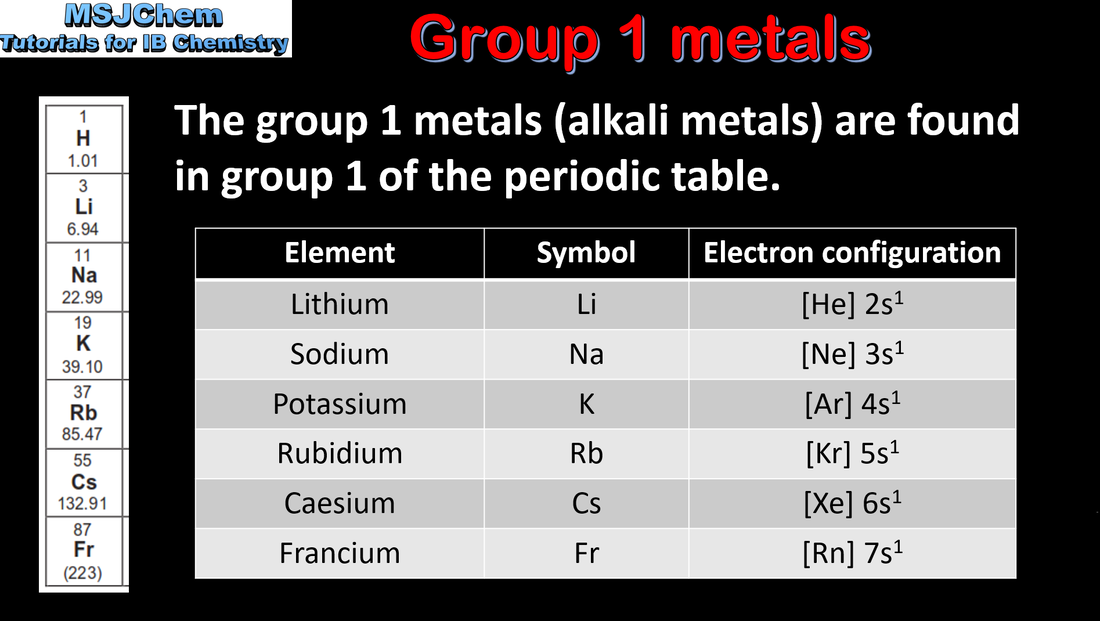
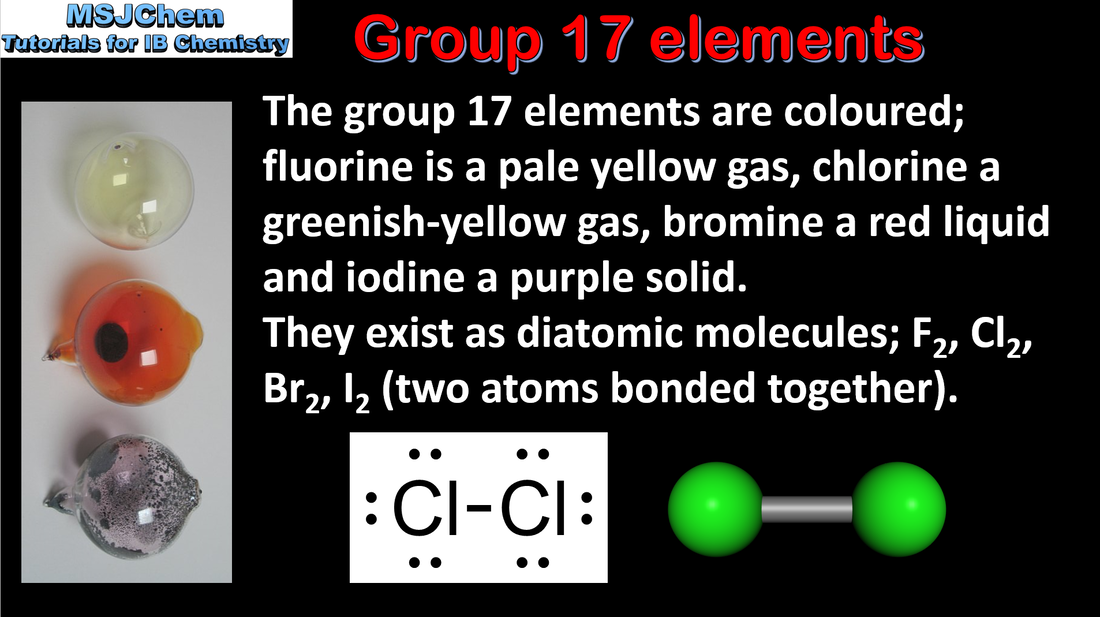
- The classifications “alkali metals”, “halogens”, “transition elements” and “noble gases” should be known.
- Structure 1.2 How has the organization of elements in the periodic table facilitated the discovery of new elements?
Structure 3.1.3
Understandings:
Understandings:
- Periodicity refers to trends in properties of elements across a period and down a group.
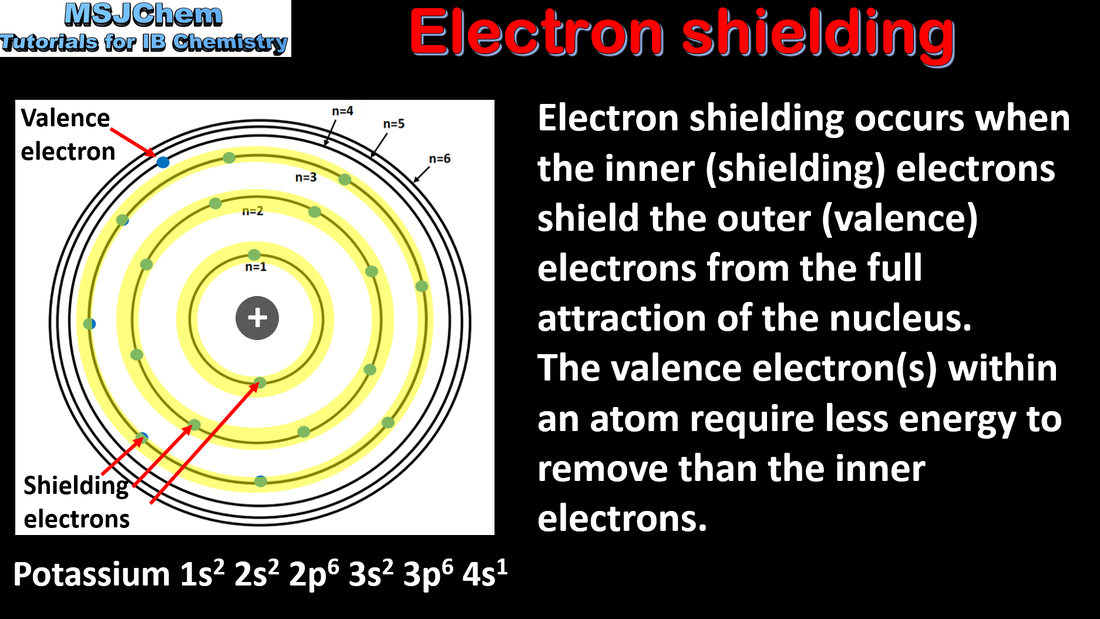
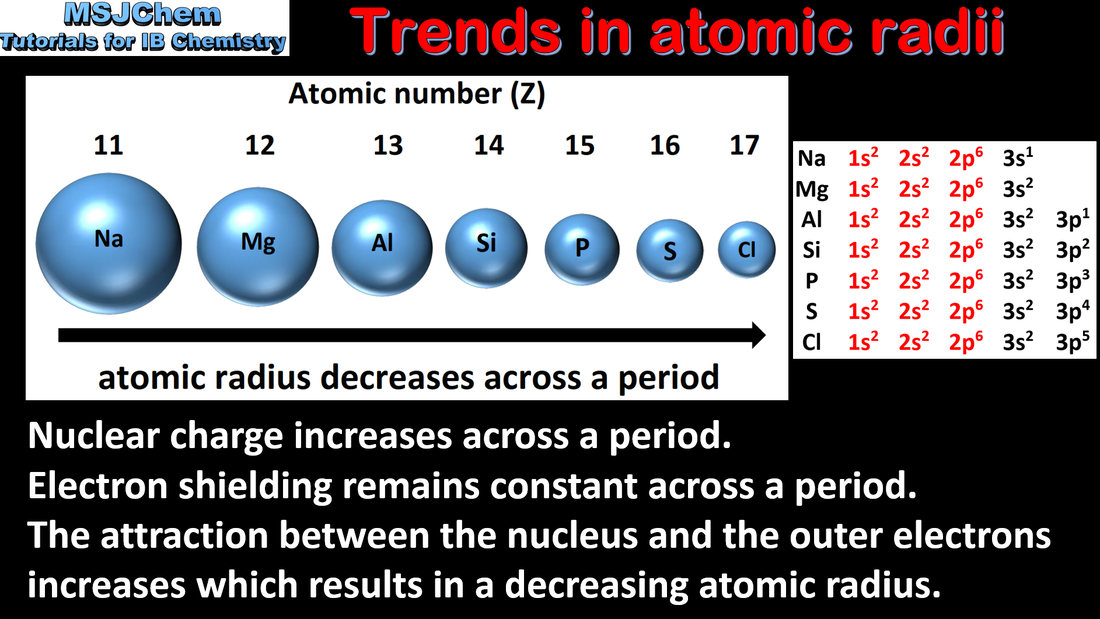
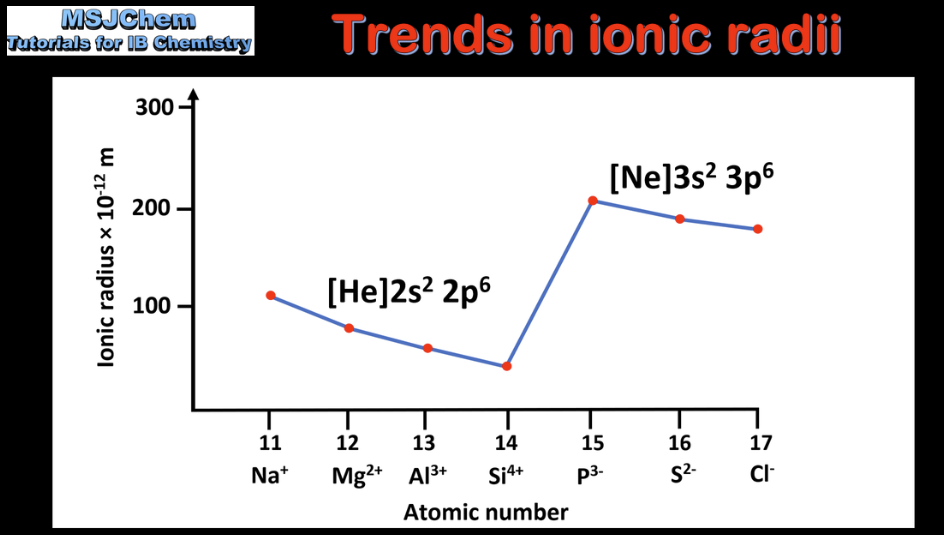
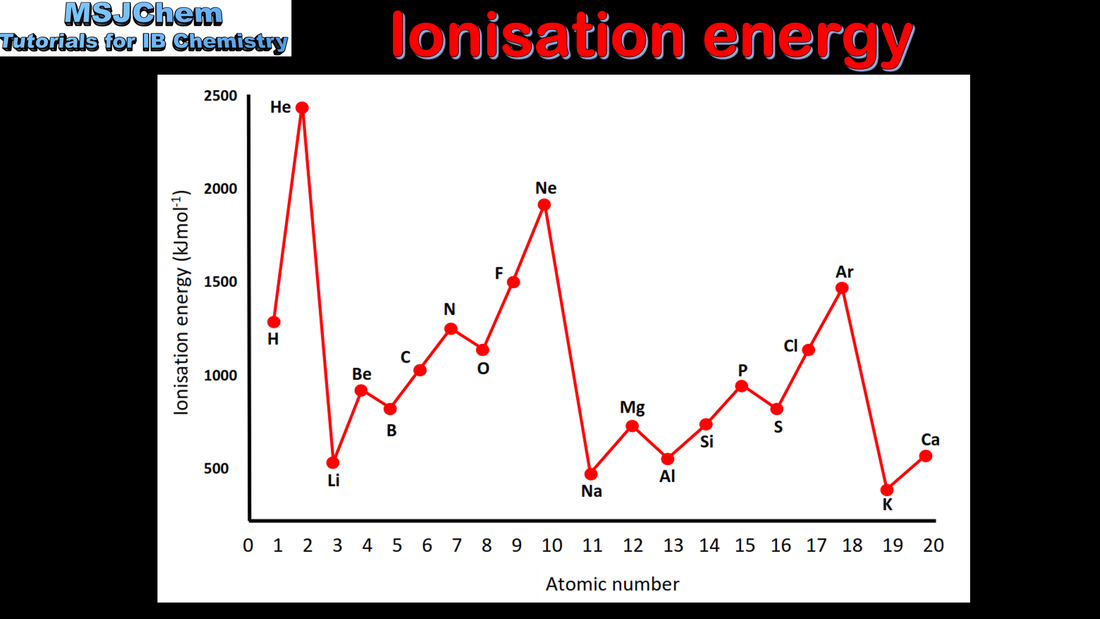
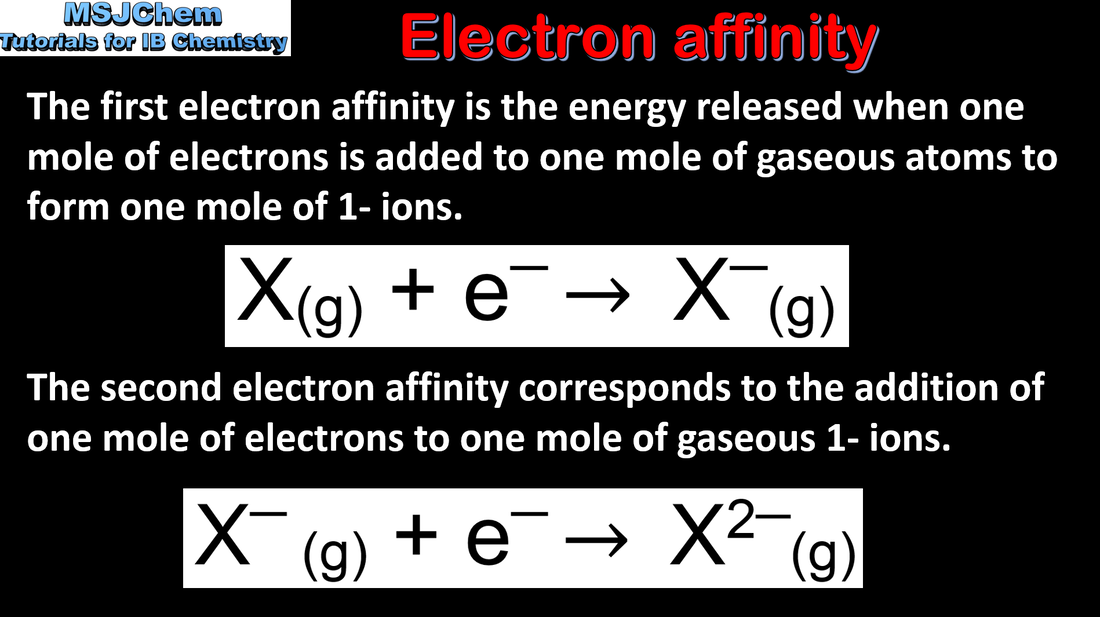
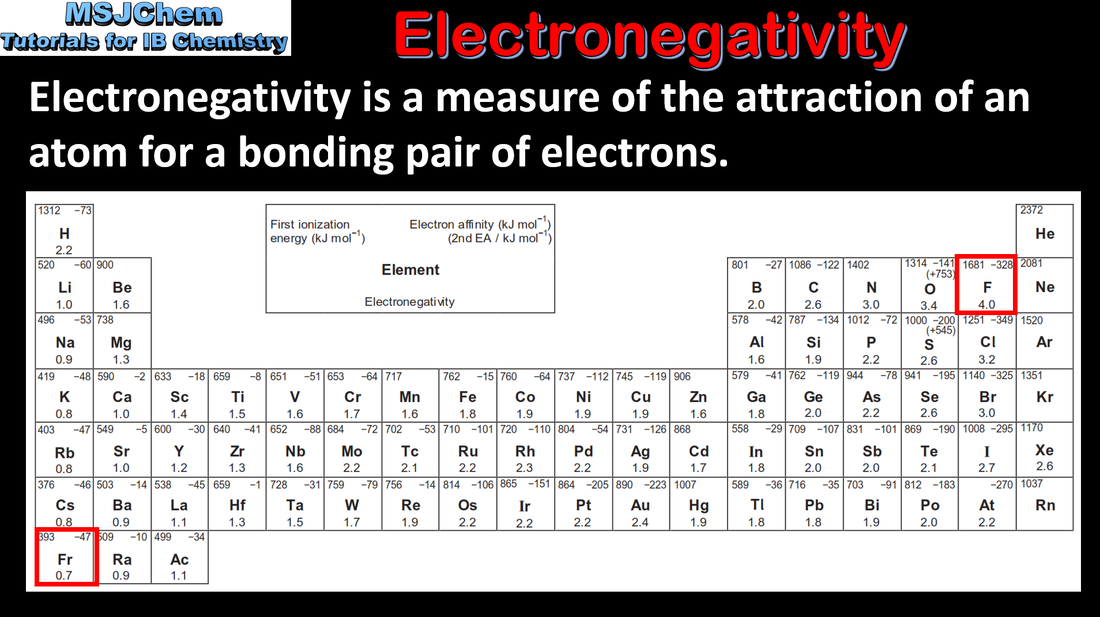
- Explain the periodicity of atomic radius, ionic radius, ionization energy, electron affinity and electronegativity.
Structure 3.1.4
Understandings:
Understandings:
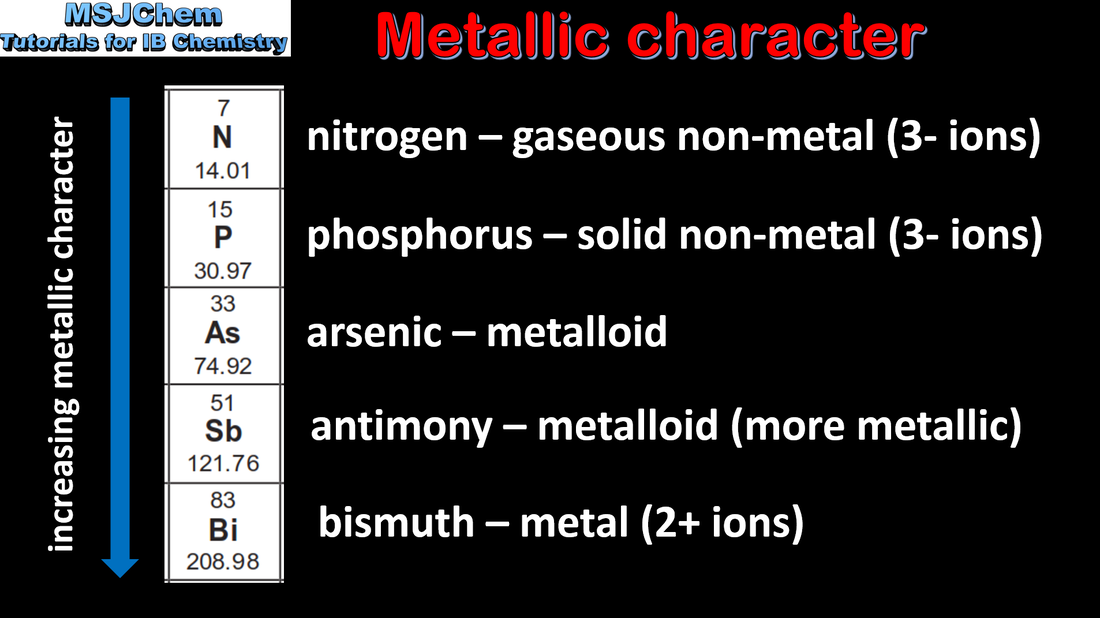
- Trends in properties of elements down a group include the increasing metallic character of group 1 elements and decreasing non-metallic character of group 17 elements.
- Describe and explain the reactions of group 1 metals with water, and of group 17 elements with halide ions.
Structure 3.1.5
Understandings:
Understandings:
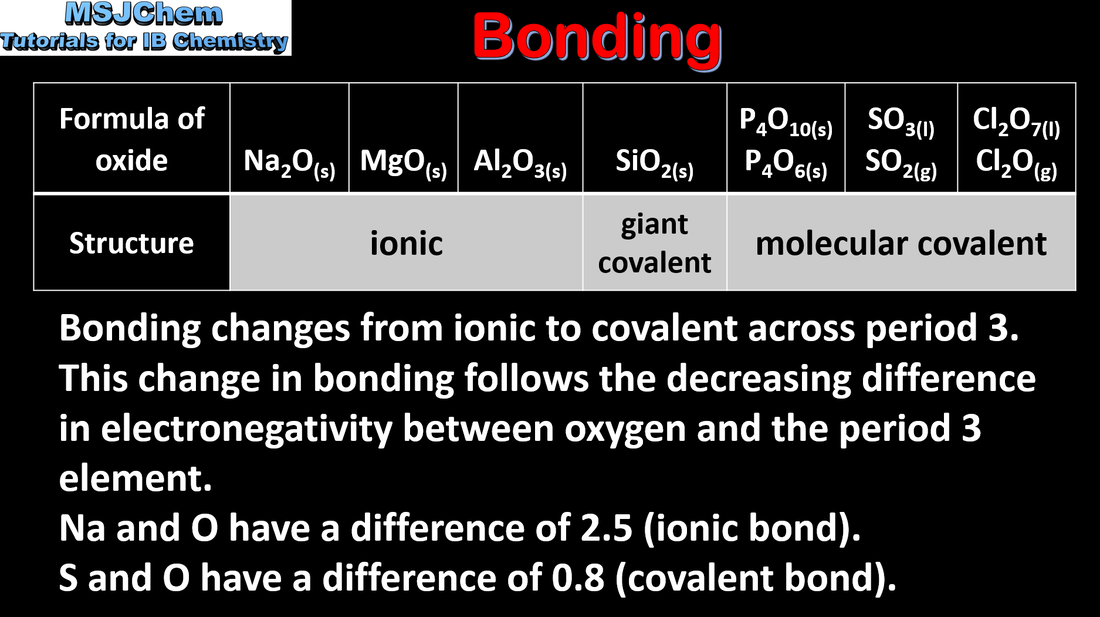
- Metallic and non-metallic properties show a continuum. This includes the trend from basic metal oxides through amphoteric to acidic non-metal oxides.
- Deduce equations for the reactions with water of the oxides of group 1 and group 2 metals, carbon and sulfur.
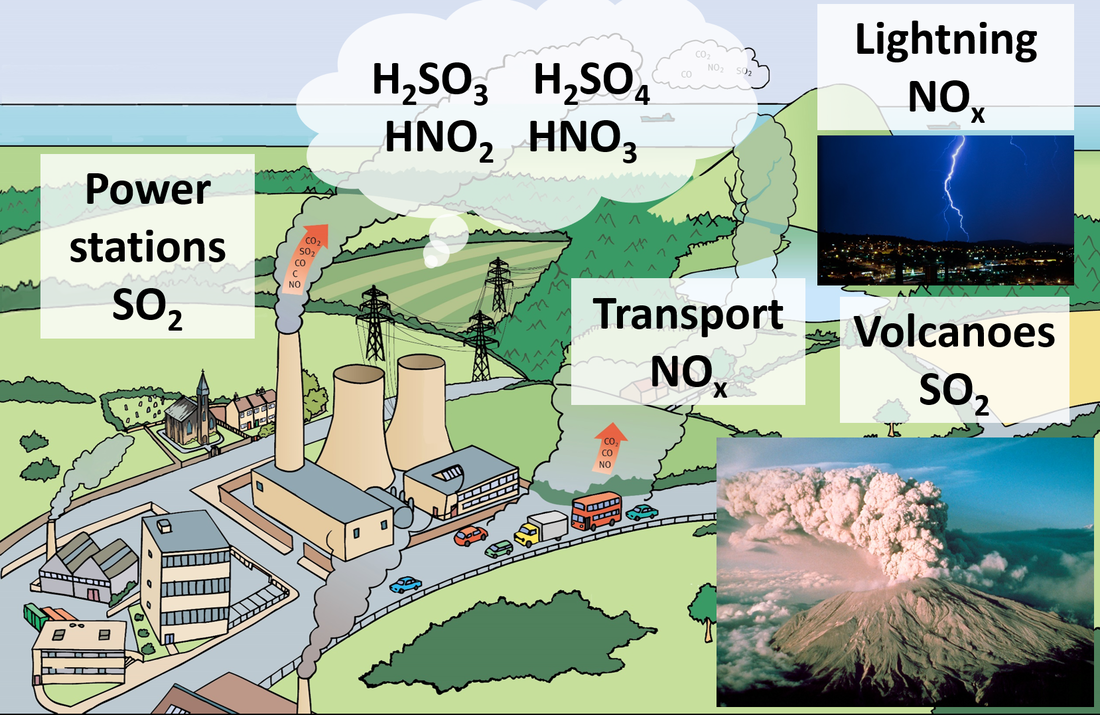
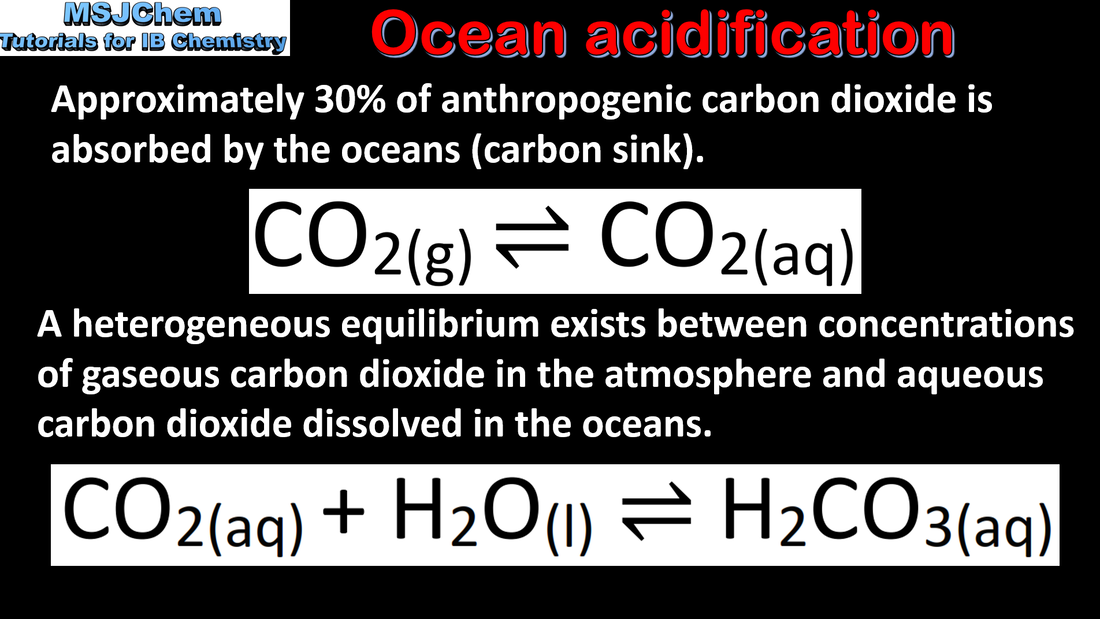
- Include acid rain caused by gaseous non-metal oxides, and ocean acidification caused by increasing CO2 levels.
- Structure 2.1, 2.2 How do differences in bonding explain the differences in the properties of metal and non-metal oxides?
Structure 3.1.6
Understandings:
Understandings:
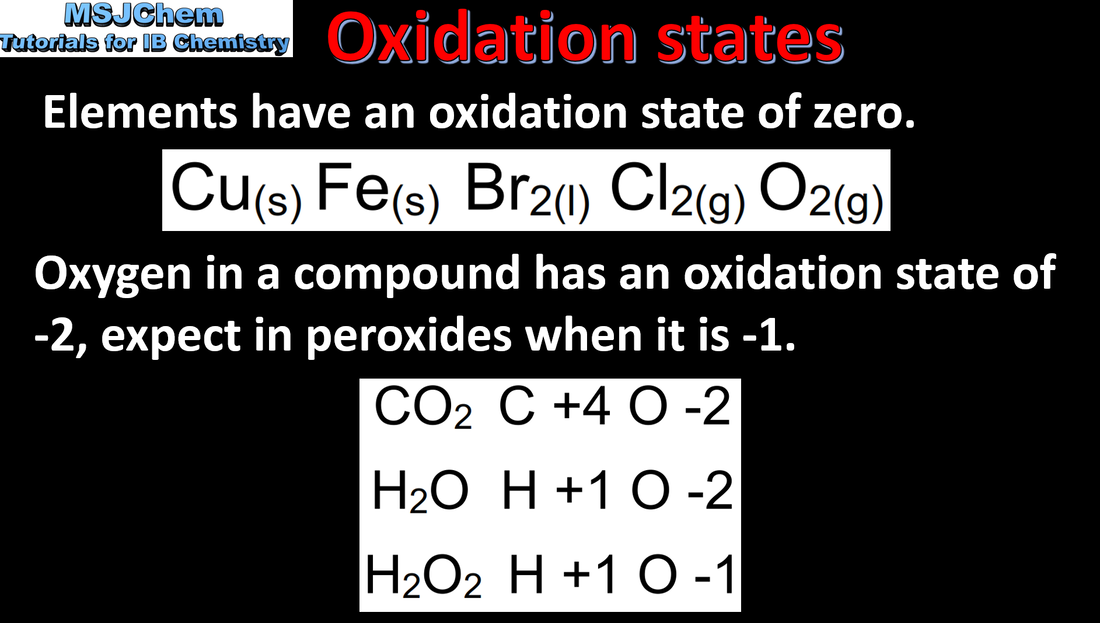
- The oxidation state is a number assigned to an atom to show the number of electrons transferred in forming a bond. It is the charge that atom would have if the compound were composed of ions.
- Deduce the oxidation states of an atom in an ion or a compound.
- Explain why the oxidation state of an element is zero.
- Oxidation states are shown with a + or – sign followed by the Arabic symbol for the number, e.g. +2, –1.
- Examples should include hydrogen in metal hydrides (–1) and oxygen in peroxides (–1).
- The terms “oxidation number” and “oxidation state” are often used interchangeably, and either term is acceptable in assessment.
- Naming conventions for oxyanions use oxidation numbers shown with Roman numerals, but generic names persist and are acceptable. Examples include nitrate, nitrite, sulfate, sulfite.
- Reactivity 3.2 How can oxidation states be used to analyse redox reactions?