Reactivity 2.2 How fast? The rate of chemical change
Reactivity 2.2.1
Understandings:
Understandings:
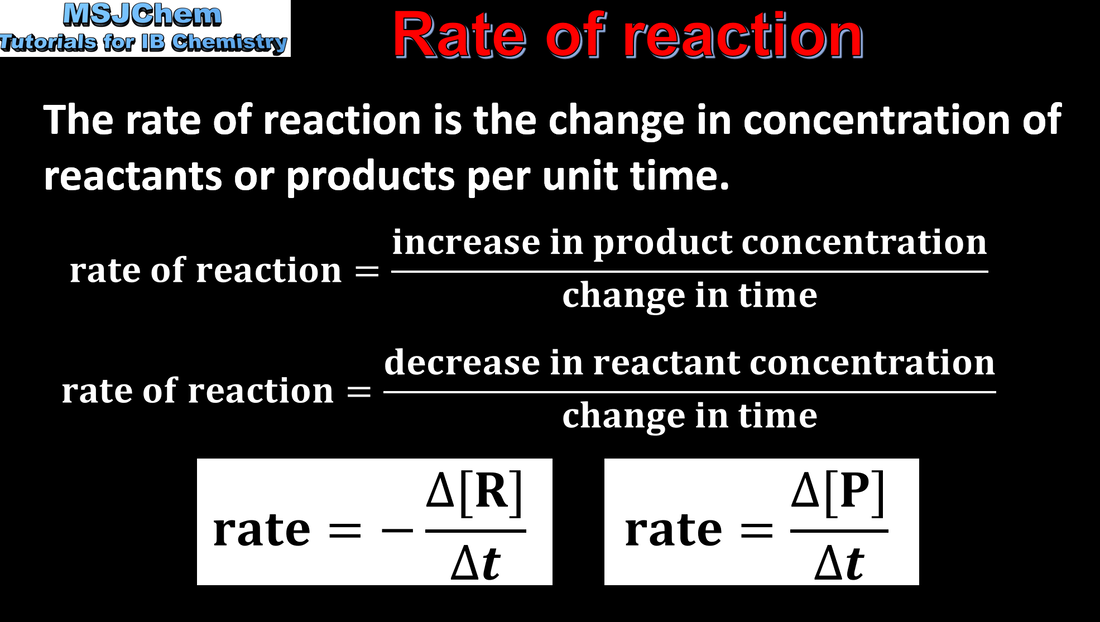
- The rate of reaction is expressed as the change in concentration of a particular reactant/product per unit time.
- Determine rates of reaction.
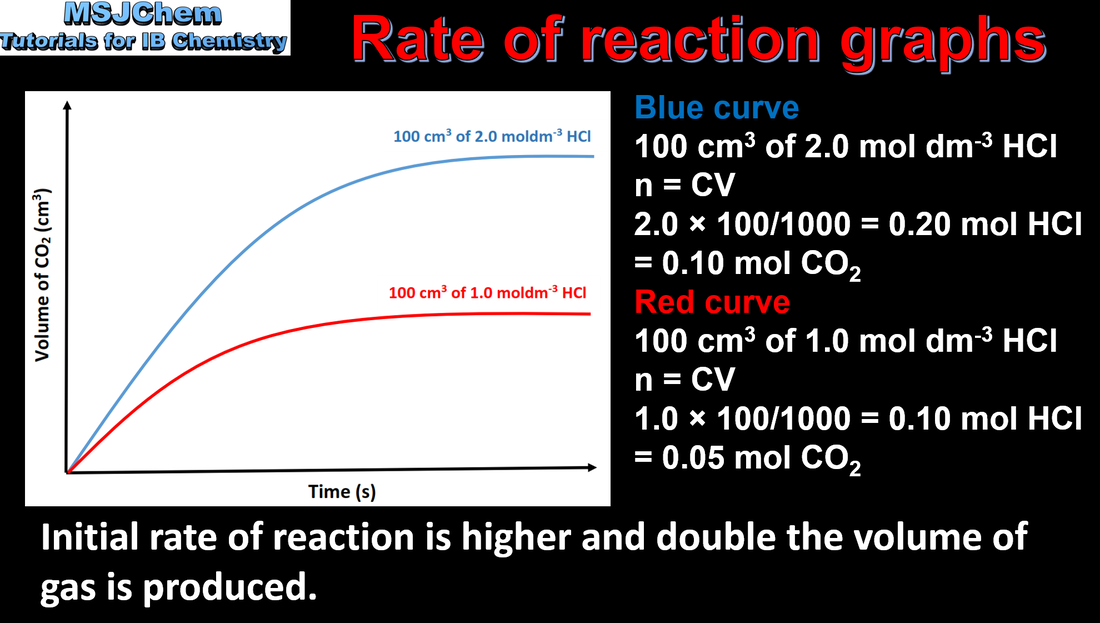
- Calculation of reaction rates from tangents of graphs of concentration, volume or mass against time should be covered.
- Tool 1, 3, Inquiry 2 Concentration changes in reactions are not usually measured directly. What methods are used to provide data to determine the rate of reactions?
- Tool 1 What experiments measuring reaction rates might use time as i) a dependent variable ii) an independent variable?
Reactivity 2.2.2
Understandings:
Understandings:
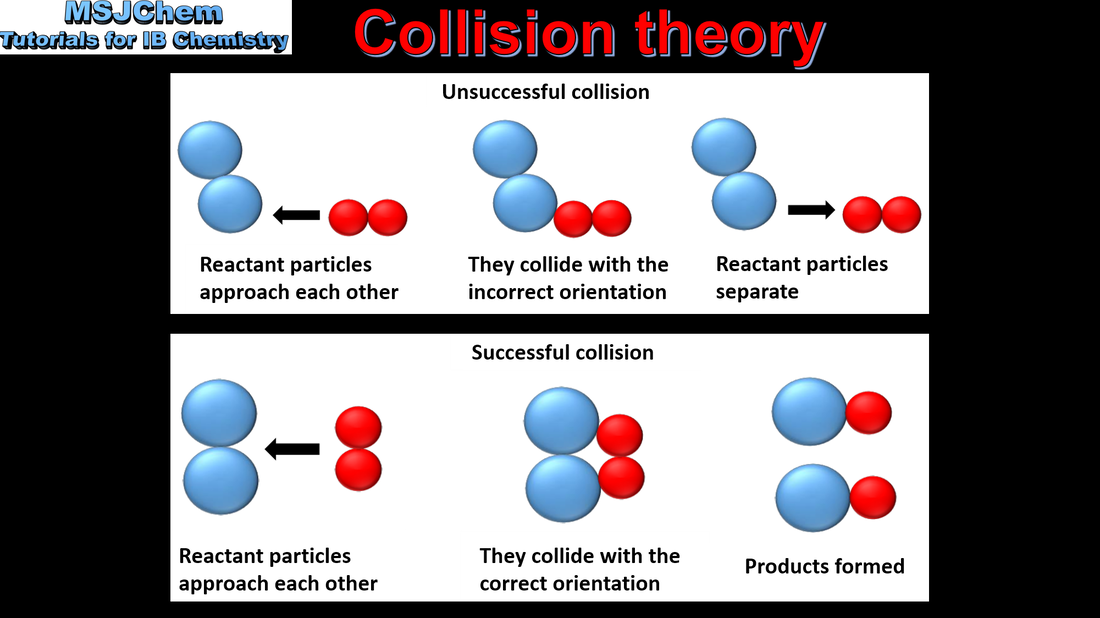
- Species react as a result of collisions of sufficient energy and proper orientation.
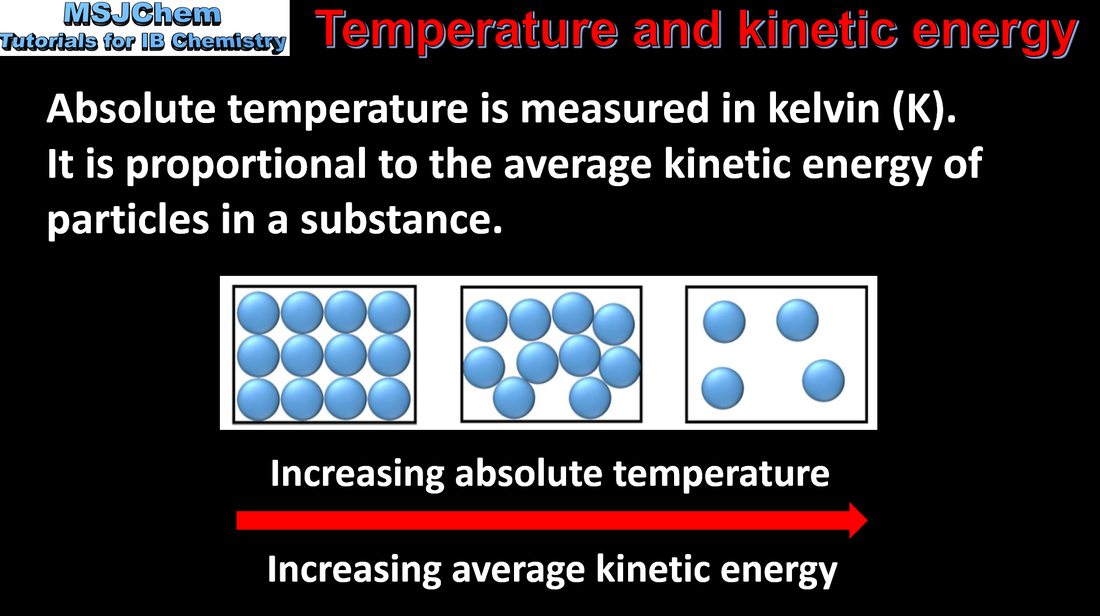
- Explain the relationship between the kinetic energy of the particles and the temperature in kelvin, and the role of collision geometry.
- Structure 1.1 What is the relationship between the kinetic molecular theory and collision theory?
Reactivity 2.2.3
Understandings:
Understandings:
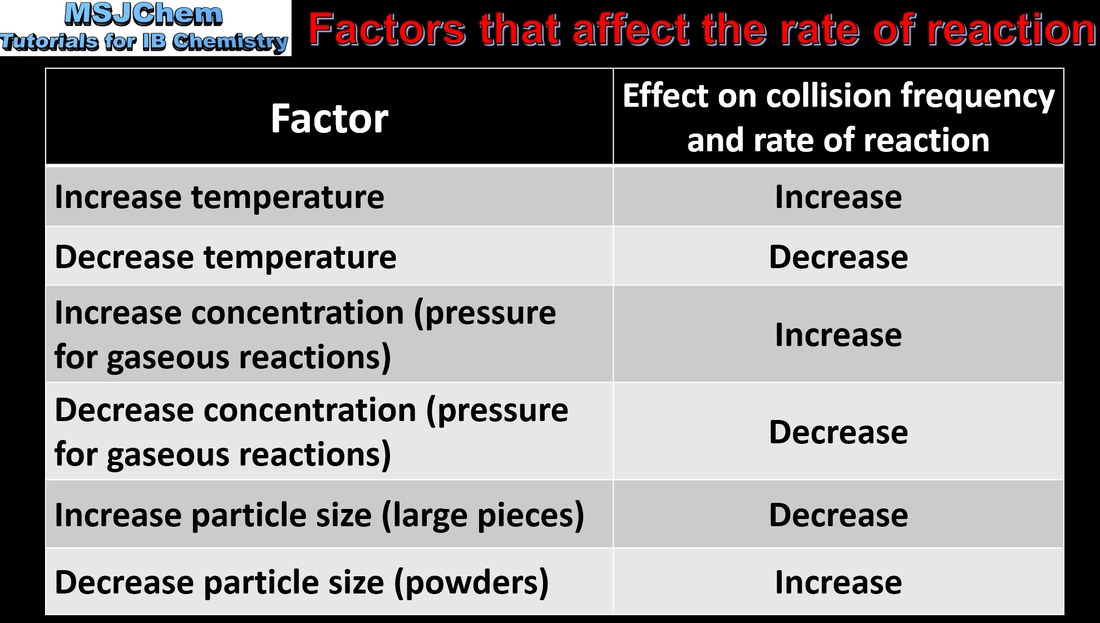
- Factors that influence the rate of a reaction include pressure, concentration, surface area, temperature and the presence of a catalyst.
- Predict and explain the effects of changing conditions on the rate of a reaction.
- Tool 1 What variables must be controlled in studying the effect of a factor on the rate of a reaction?
- Nature of science, Tool 3, Inquiry 3 How can graphs provide evidence of systematic and random error?
Reactivity 2.2.4
Understandings:
Understandings:
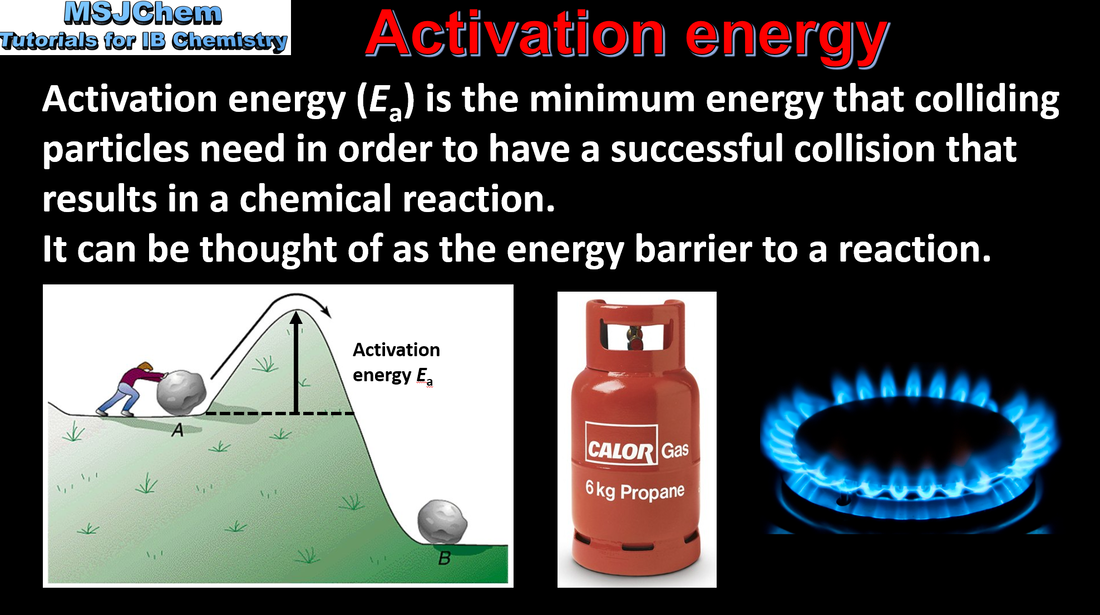
- Activation energy, Ea, is the minimum energy that colliding particles need for a successful collision leading to a reaction.
- Construct Maxwell–Boltzmann energy distribution curves to explain the effect of temperature on the probability of successful collisions.
Reactivity 2.2.5
Understandings:
Understandings:
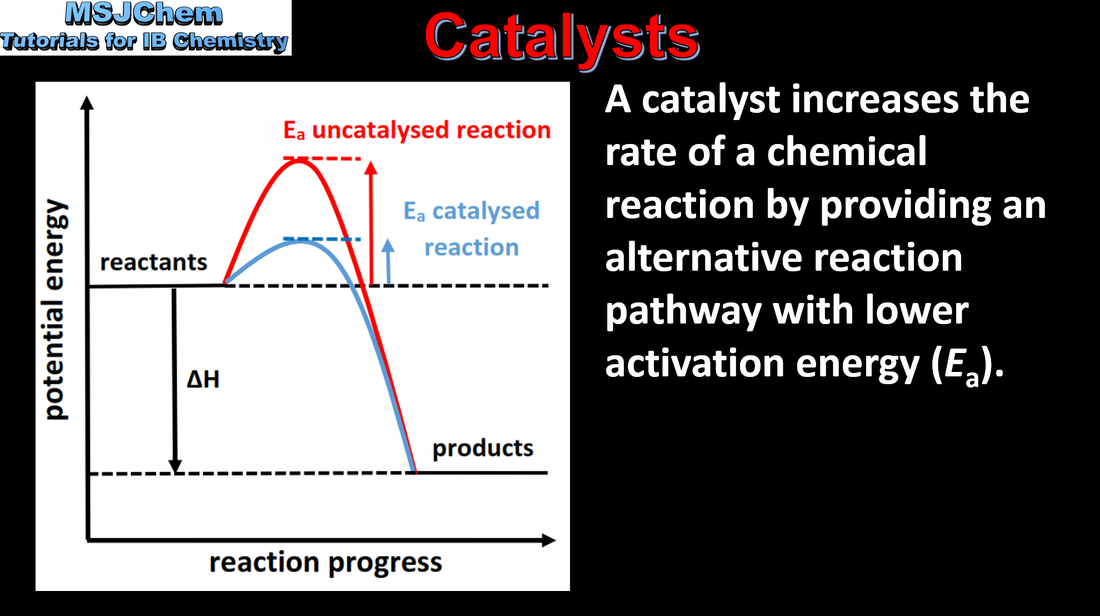
- Catalysts increase the rate of reaction by providing an alternative reaction pathway with lower Ea.
- Sketch and explain energy profiles with and without catalysts for endothermic and exothermic reactions.
- Construct Maxwell–Boltzmann energy distribution curves to explain the effect of different values for Ea on the probability of successful collisions.
- Biological catalysts are called enzymes.
- The different mechanisms of homogeneous and heterogeneous catalysts will not be assessed.
- Reactivity 2.3 What is the relative effect of a catalyst on the rate of the forward and backward reactions?
- Structure 3.1 (HL) What are the features of transition elements that make them useful as catalysts?