Reactivity 2.3 How far? The extent of chemical change
Reactivity 2.3.1
Understandings:
Understandings:
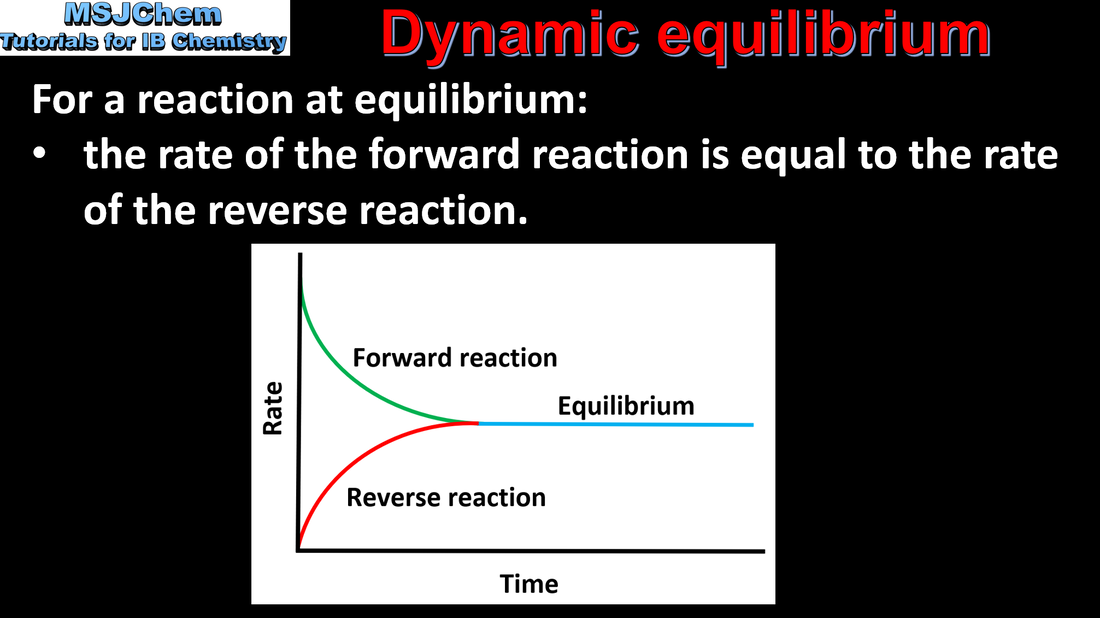
- A state of dynamic equilibrium is reached in a closed system when the rates of forward and backward reactions are equal.
- Describe the characteristics of a physical and chemical system at equilibrium.
Reactivity 2.3.2 and 2.2.3
Understandings:
Linking questions:
Understandings:
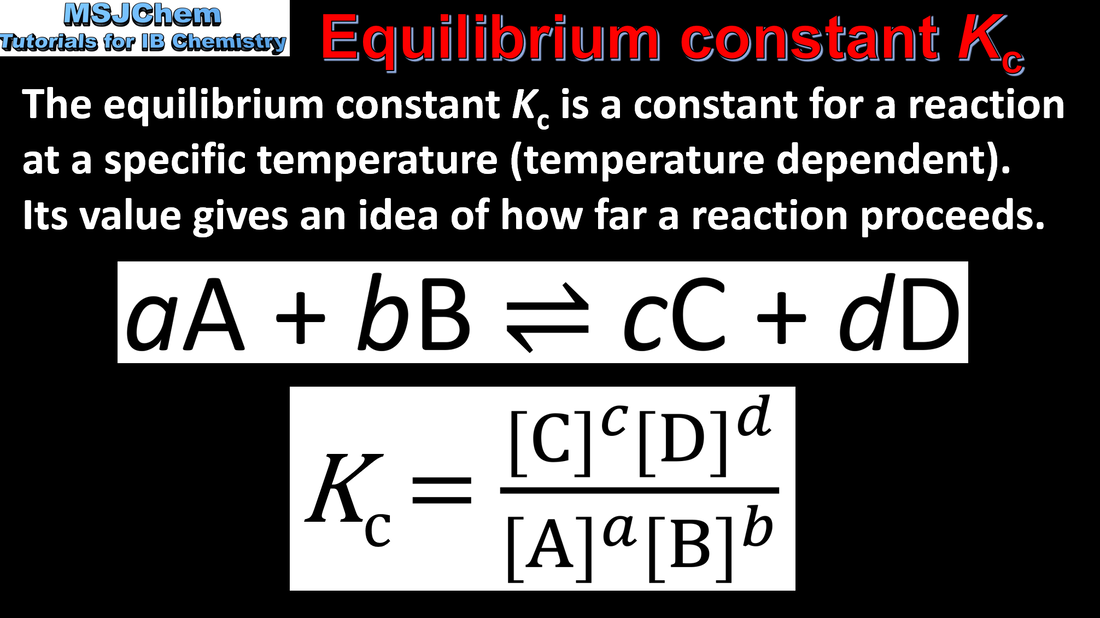
- The equilibrium law describes how the equilibrium constant, K, can be determined from the stoichiometry of a reaction (2.2.2).
- The magnitude of the equilibrium constant indicates the extent of a reaction at equilibrium and is temperature dependent (2.2.3).
- Deduce the equilibrium constant expression from an equation for a homogeneous reaction (2.2.2).
- Determine the relationships between K values for reactions that are the reverse of each other at the same temperature (2.2.3).
- Include the extent of reaction for:
Linking questions:
- Reactivity 3.1 How does the value of K for the dissociation of an acid convey information about its strength?
Reactivity 2.3.4
Understandings:
Understandings:
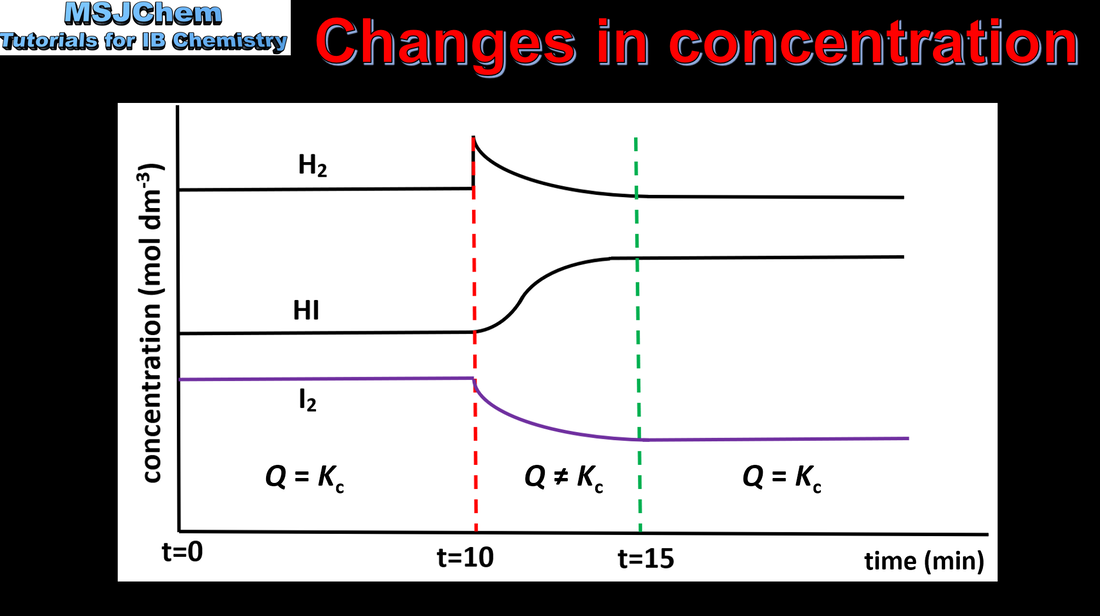
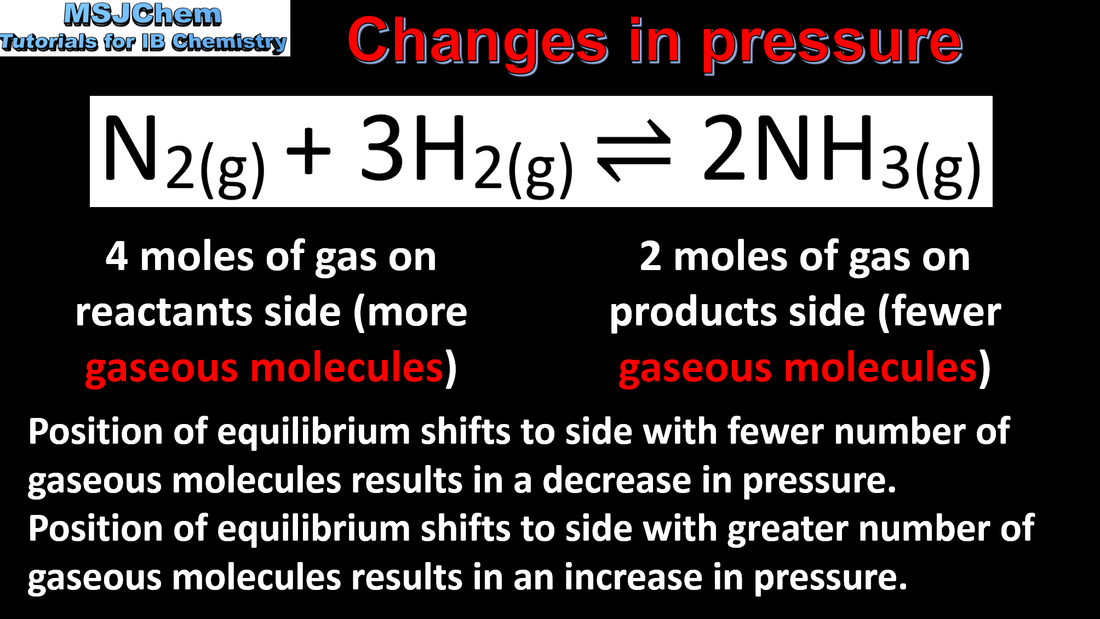
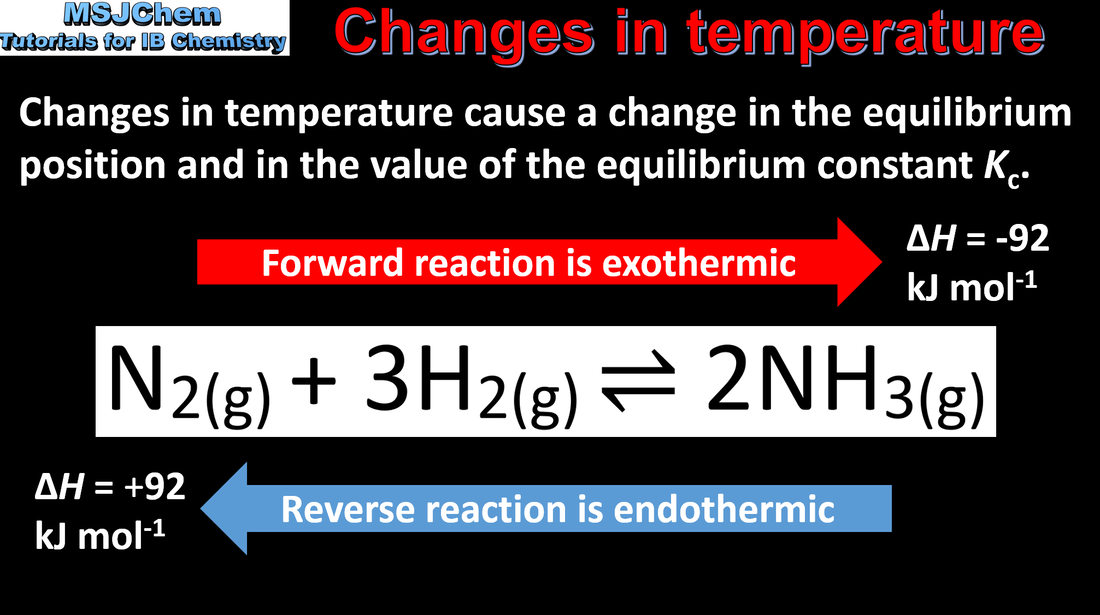
- Le Châtelier’s principle enables the prediction of the qualitative effects of changes in concentration, temperature and pressure to a system at equilibrium.
- Apply Le Châtelier’s principle to predict and explain responses to changes of systems at equilibrium.
- Include the effects on the value of K and on the equilibrium composition.
- Le Châtelier’s principle can be applied to heterogeneous equilibria such as: X(g) ⇌ X(aq).
- Reactivity 2.2 Why do catalysts have no effect on the value of K or on the equilibrium composition?