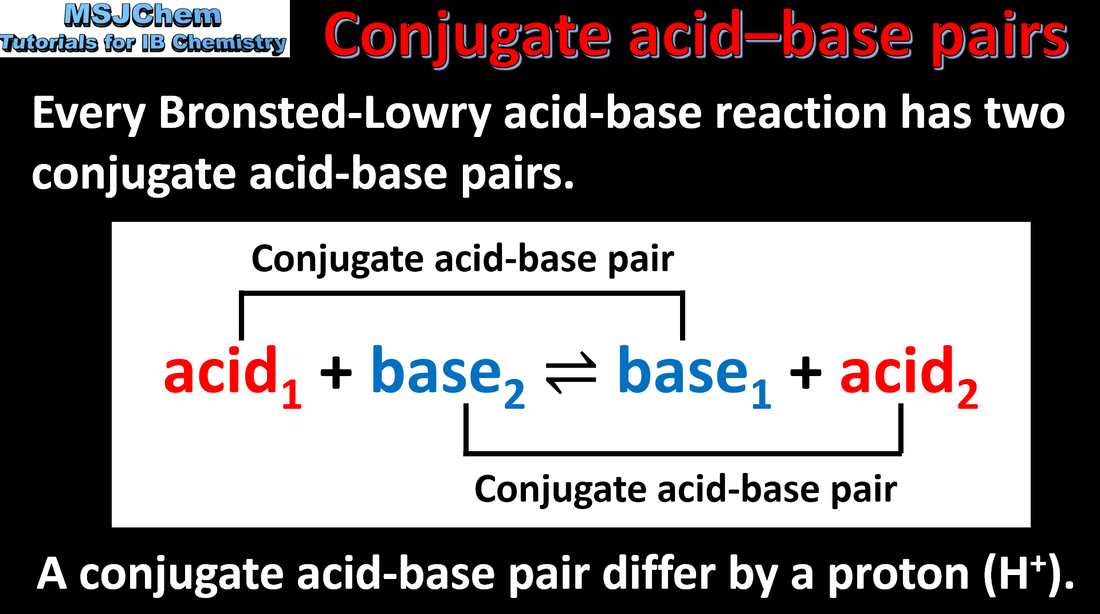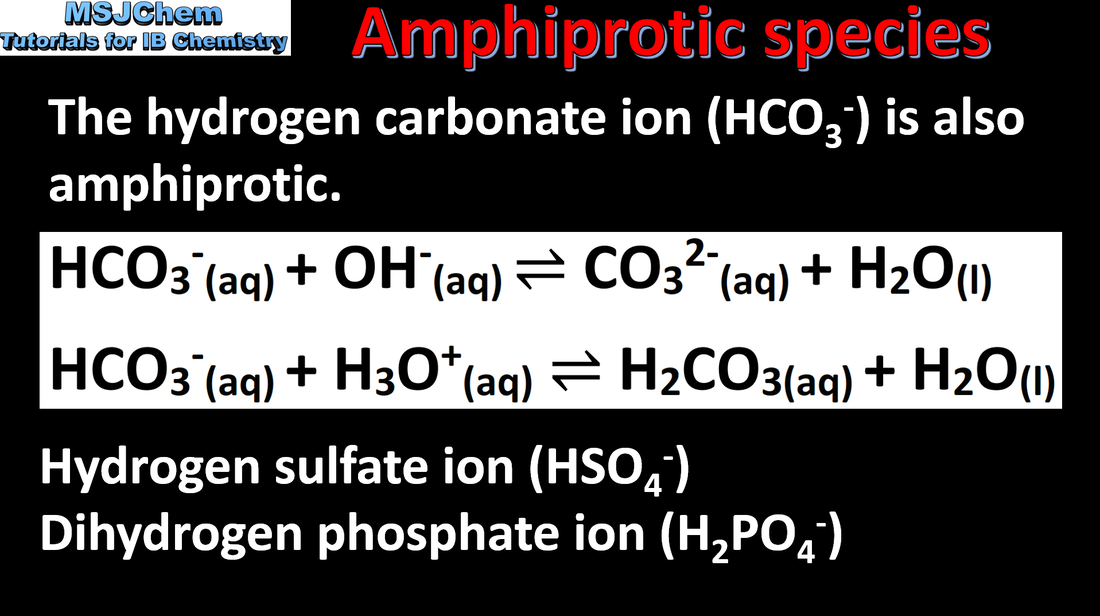Reactivity 3.1 Proton transfer reactions
Reactivity 3.1.1
Understandings:
Understandings:
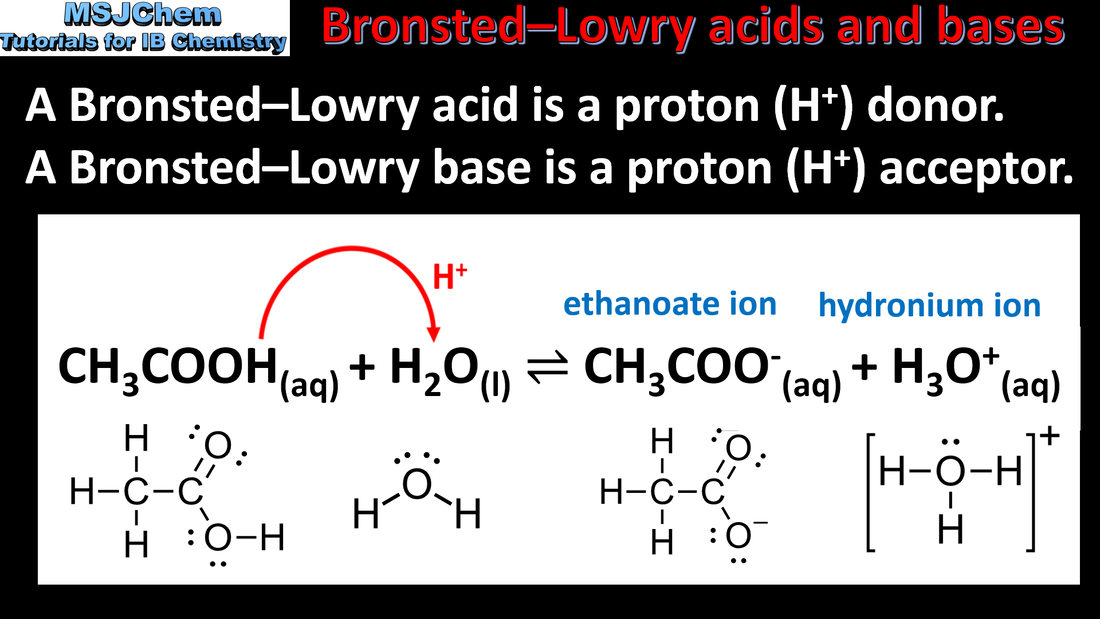
- Brønsted–Lowry acid is a proton donor and a Brønsted–Lowry base is a proton acceptor.
- Deduce the Brønsted–Lowry acid and base in a reaction.
- A proton in aqueous solution can be represented as both H+(aq) and H3O+(aq). The distinction between the terms “base” and “alkali” should be understood.
Reactivity 3.1.2
Understandings:
Understandings:
- A pair of species differing by a single proton is called a conjugate acid–base pair.
- Deduce the Brønsted–Lowry acid and base in a reaction.
- Structure 2.1 What are the conjugate acids of the polyatomic anions listed in Structure 2.1?
Reactivity 3.1.3
Understandings:
Understandings:
- Some species can act as both Brønsted–Lowry acids and bases.
- Interpret and formulate equations to show acid–base reactions of these species.
- Structure 3.1 What is the periodic trend in the acid–base properties of metal and non-metal oxides?
- Structure 3.1 Why does the release of oxides of nitrogen and sulfur into the atmosphere cause acid rain?
Reactivity 3.1.4
Understandings:
Understandings:
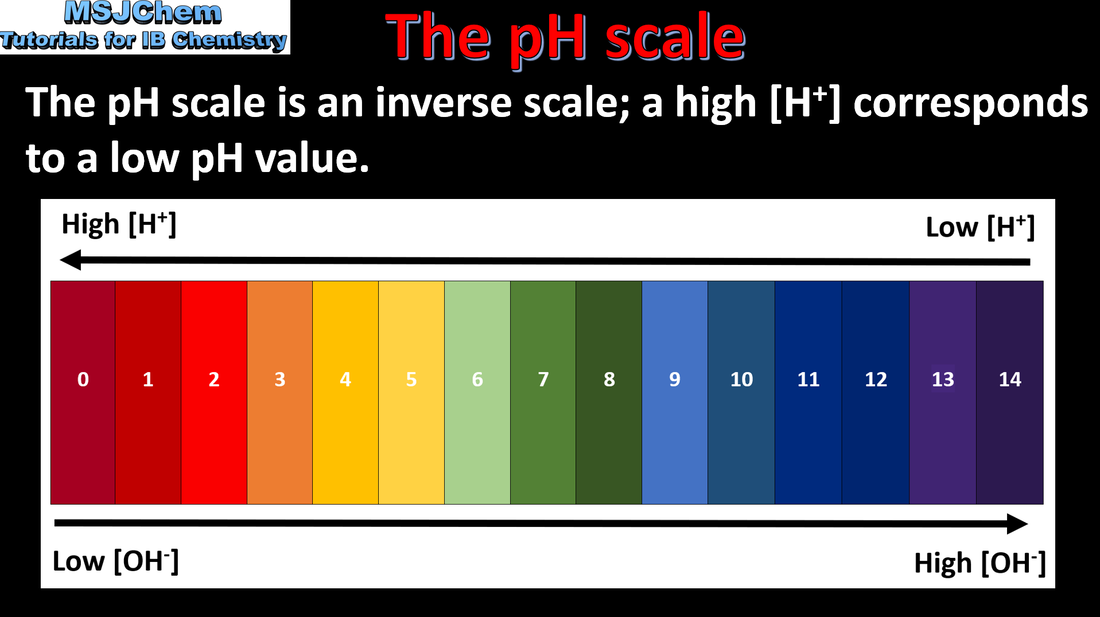
- The pH scale can be used to describe the [H+] of a solution: pH = –log10[H+]; [H+] = 10–pH
- Perform calculations involving the logarithmic relationship between pH and [H+].
- Include the estimation of pH using universal indicator, and the precise measurement of pH using a pH meter/probe.
- The equations for pH are given in the data booklet.
Reactivity 3.1.5
Understandings:
Understandings:
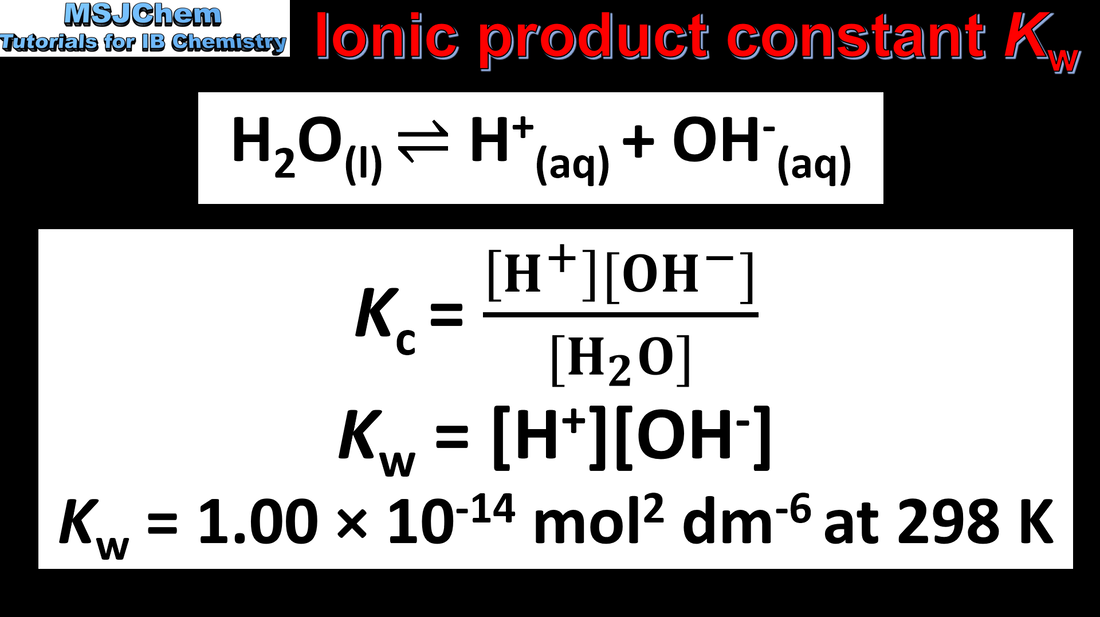
- The ion product constant of water, Kw, shows an inverse relationship between [H+] and [OH–]. Kw = [H+] [OH–]
- Recognize solutions as acidic, neutral and basic from the relative values of [H+] and [OH–].
- The equation for Kw and its value at 298 K are given in the data booklet.
- Reactivity 2.3 Why does the extent of ionization of water increase as temperature increases?
Reactivity 3.1.6
Understandings:
Understandings:
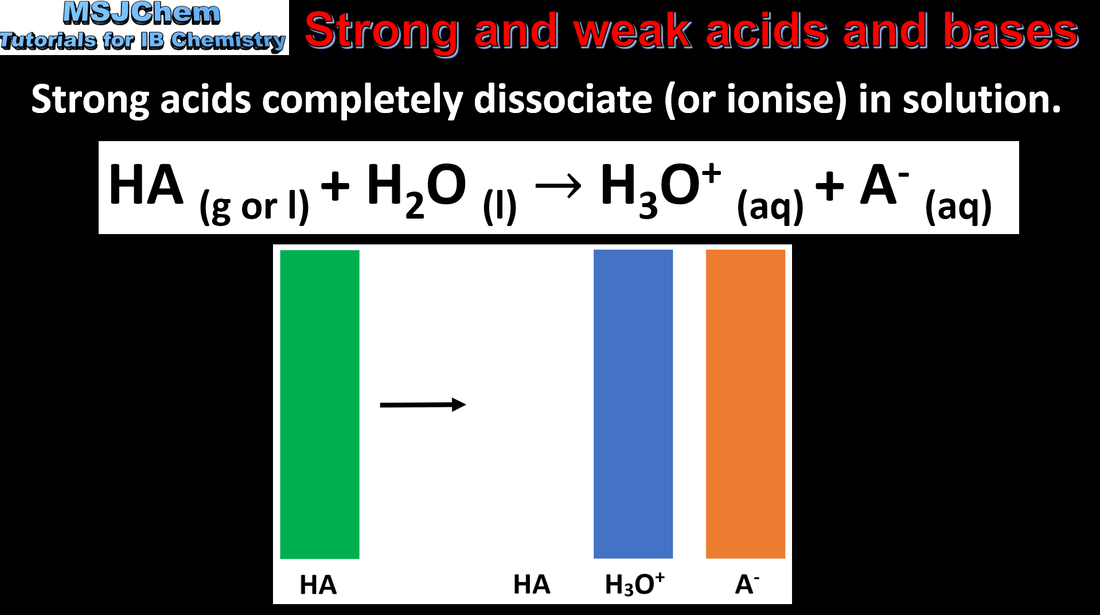
- Strong and weak acids and bases differ in the extent of ionisation.
- Recognise that acid–base equilibria lie in the direction of the weaker conjugate.
- HCl, HBr, HI, H2SO4 and HCl are strong acids, and group 1 hydroxides are strong bases.
- The distinction between strong and weak acids or bases and concentrated and dilute reagents should be covered.
- Reactivity 2.3 How would you expect the equilibrium constants of strong and weak acids to compare?
- Reactivity 1.1 Why does the acid strength of the hydrogen halides increase down group 17?
Reactivity 3.1.7
Understandings:
Understandings:
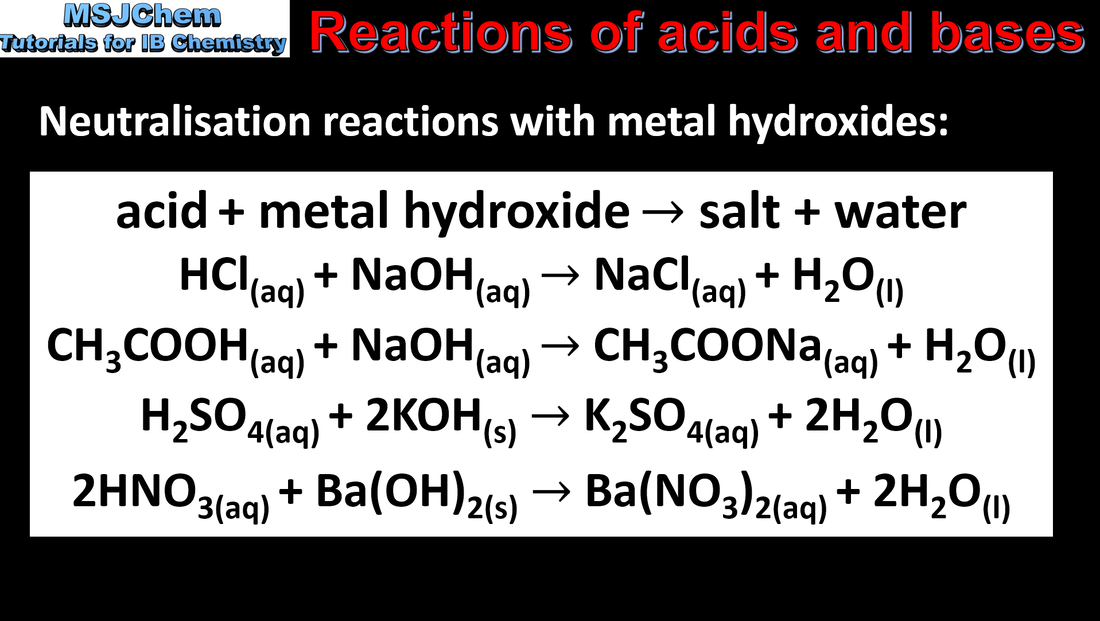
- Acids react with bases in neutralisation reactions.
- Formulate equations for the reactions between acids and metal oxides, metal hydroxides, hydrogencarbonates and carbonates.
- Identify the parent acid and base of different salts.
- Bases should include ammonia, amines, soluble carbonates and hydrogencarbonates; acids should include organic acids.
- Reactivity 1.1 Neutralisation reactions are exothermic. How can this be explained in terms of bond enthalpies?
- Reactivity 3.2 How could we classify the reaction that occurs when hydrogen gas is released from the reaction between an acid and a metal?
Reactivity 3.1.8
Understandings:
Understandings:
- pH curves for neutralization reactions involving strong acids and bases have characteristic shapes and features.
- Sketch and interpret the general shape of the pH curve.
- Interpretation should include the intercept with the pH axis and equivalence point.
- Only monoprotic neutralization reactions will be assessed.
- Structure 1.4 Why is the equivalence point sometimes referred to as the stoichiometric point?
|
Video coming soon.
|