Structure 2.1 The ionic model
Structure 2.1.1
Understandings:
Understandings:
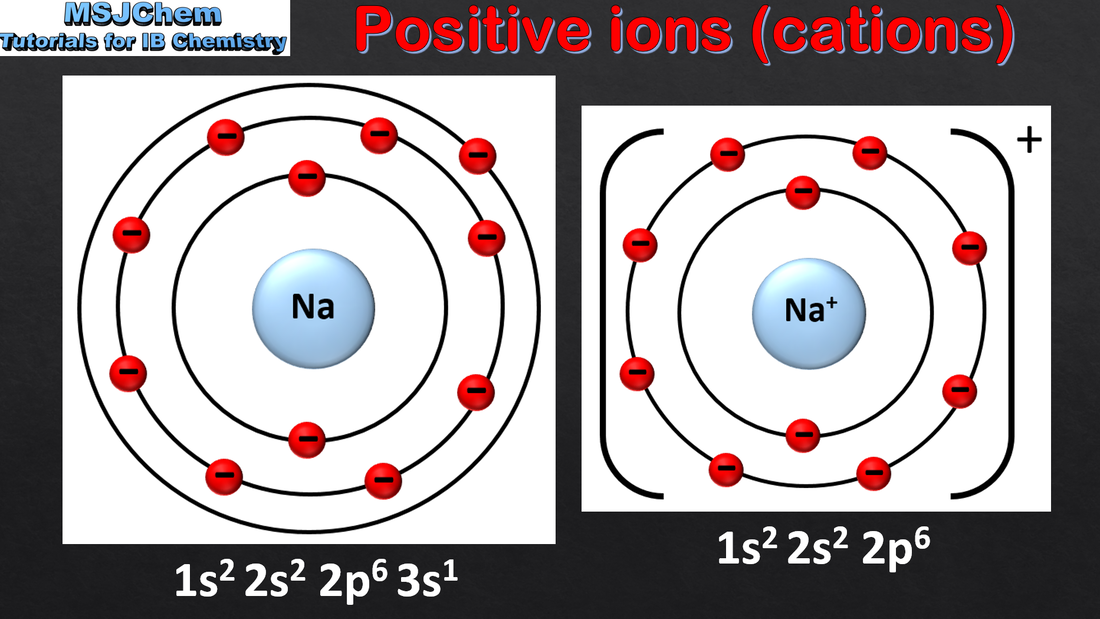
- When metal atoms lose electrons, they form positive ions called cations. When non-metal atoms gain electrons, they form negative ions called anions.
- Predict the charge of an ion from the electron configuration of the atom.
- The formation of ions with different charges from a transition element should be included.
- Structure 3.1 How does the position of an element in the periodic table relate to the charge of its ion(s)
- Structure 1.3 (HL) How does the trend in successive ionisation energies of transition elements explain their variable oxidation states?
Structure 2.1.2
Understandings:
Understandings:
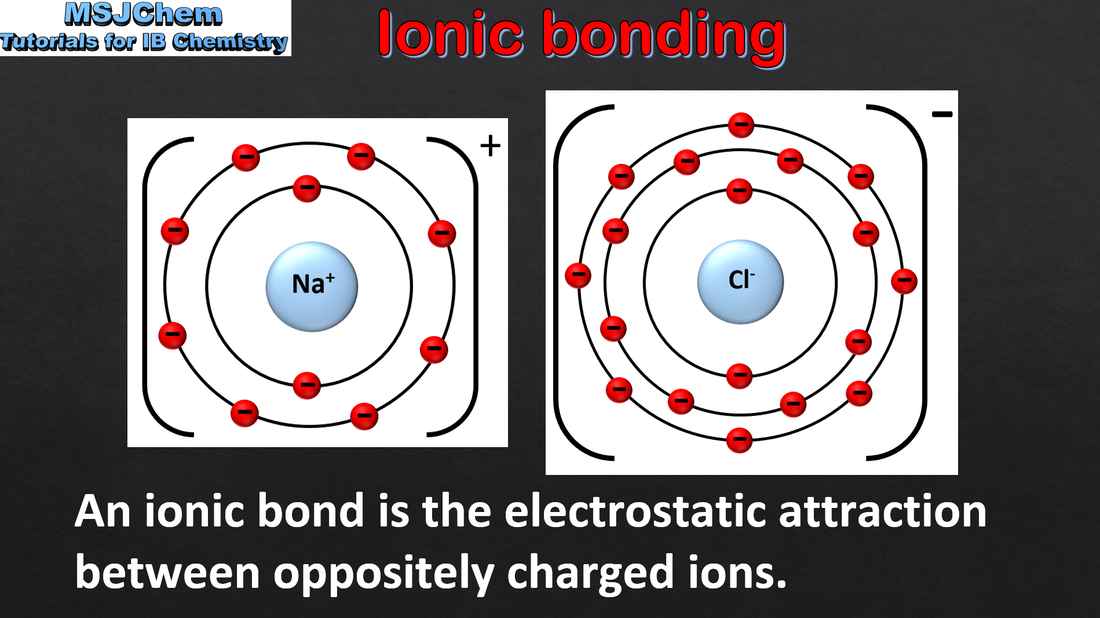
- The ionic bond is formed by electrostatic attractions between oppositely charged ions.
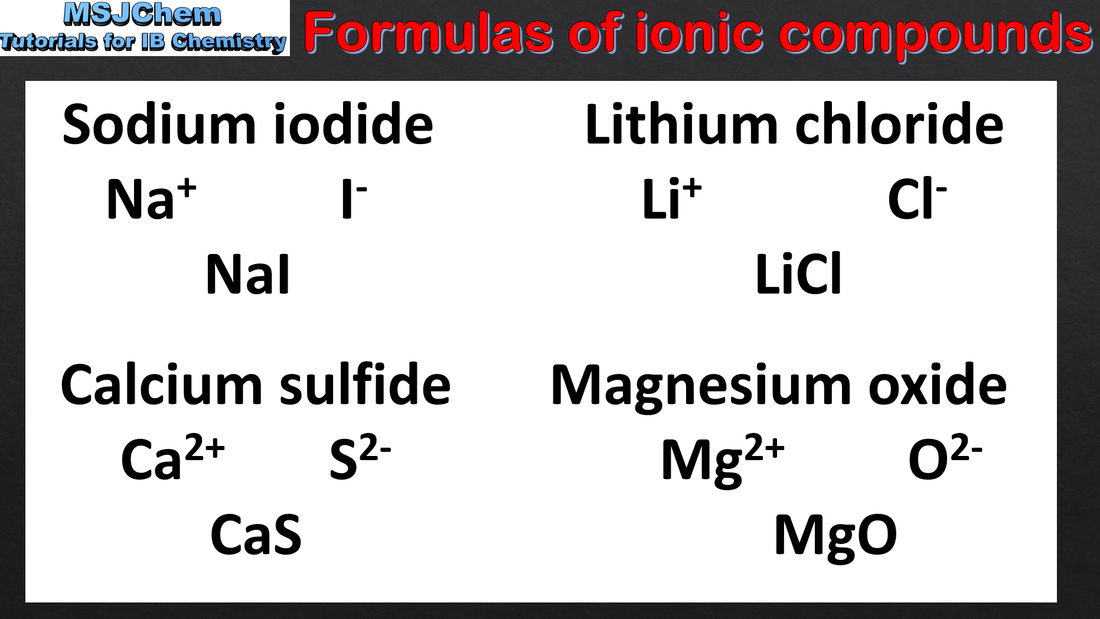
- Binary ionic compounds are named with the cation first, followed by the anion. The anion adopts the suffix “ide”.
- Deduce the formula and name of an ionic compound from its component ions, including polyatomic ions.
- Interconvert names and formulas of binary ionic compounds.
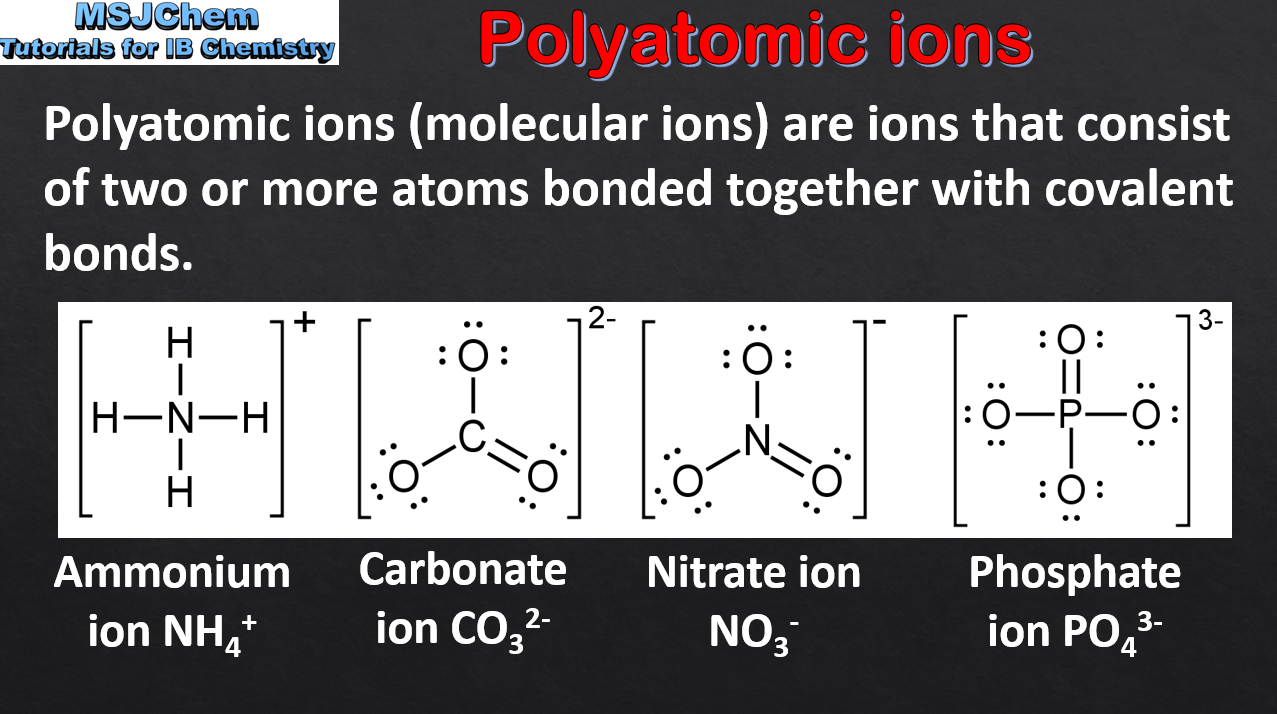
- The following polyatomic ions should be known by name and formula: ammonium, hydroxide, nitrate, hydrogencarbonate, carbonate, sulfate and phosphate.
- Reactivity 3.2 Why is the formation of an ionic compound from its elements a redox reaction?
- HL Structure 2.2 How is formal charge used to predict the preferred structure of sulfate?
- HL Reactivity 3.1 Polyatomic anions are conjugate bases of common acids. What is the relationship between their stability and the conjugate acid’s dissociation constant, Ka?
Structure 2.1.3
Understandings:
Understandings:
- Ionic compounds exist as three-dimensional lattice structures, represented by empirical formulas.
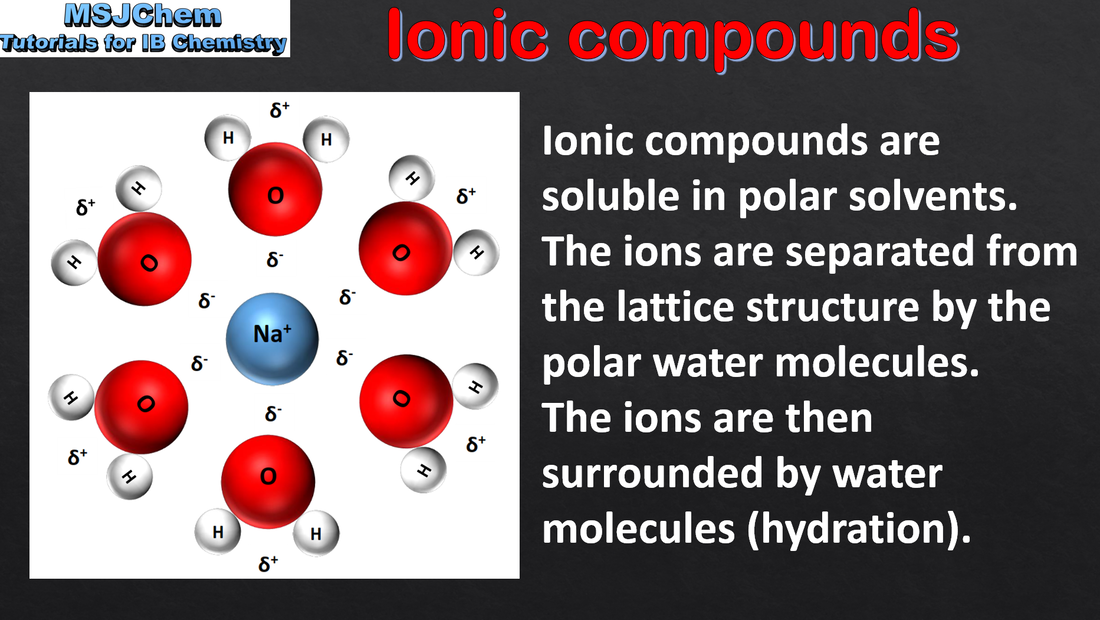
- Explain the physical properties of ionic compounds to include volatility, electrical conductivity and solubility.
- Include lattice enthalpy as a measure of the strength of the ionic bond in different compounds, influenced by ion radius and charge.
- Structure 3.1 How can lattice enthalpies and the bonding continuum explain the trend in melting points of metal chlorides across period 3?