Reactivity 3.4 Electron-pair sharing reactions
Reactivity 3.4.1
Understandings:
Understandings:
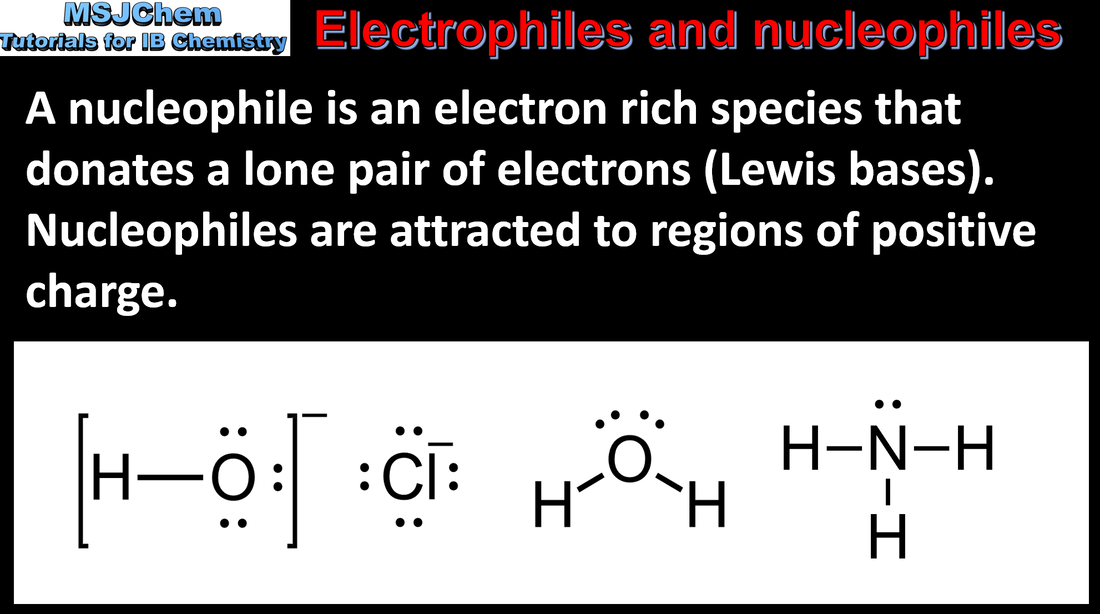
- A nucleophile is a reactant that forms a bond to its reaction partner (the electrophile) by donating both bonding electrons.
- Recognise nucleophiles in chemical reactions.
- Both neutral and negatively charged species should be included.
Reactivity 3.4.2
Understandings:
Understandings:
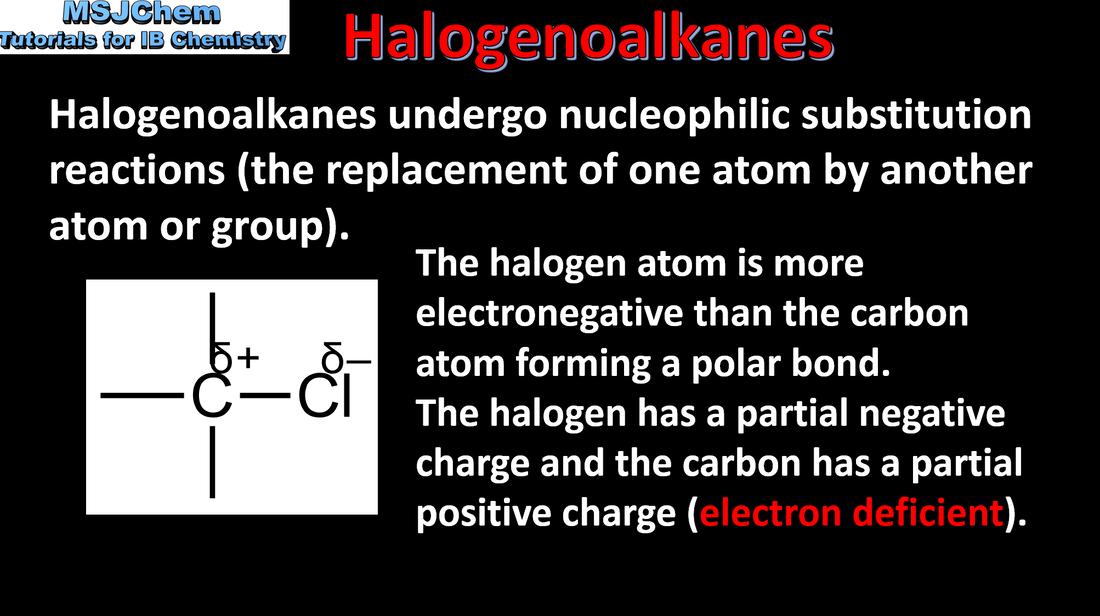
- In a nucleophilic substitution reaction, a nucleophile donates an electron pair to form a new bond, as another bond breaks producing a leaving group.
- Deduce equations with descriptions and explanations of the movement of electron pairs in nucleophilic substitution reactions.
- Further details of the mechanisms are not required at SL.
Reactivity 3.4.3
Understandings:
Understandings:
- Heterolytic fission is the breakage of a covalent bond when both bonding electrons remain with one of the two fragments formed.
- Explain, with equations, the formation of ions by heterolytic fission.
- Curly arrows should be used to show the movement of electron pairs during reactions.
- Reactivity 3.3 What is the difference between the bond-breaking that forms a radical and the bond- breaking that occurs in nucleophilic substitution reactions?
Reactivity 3.4.4
Understandings:
Understandings:
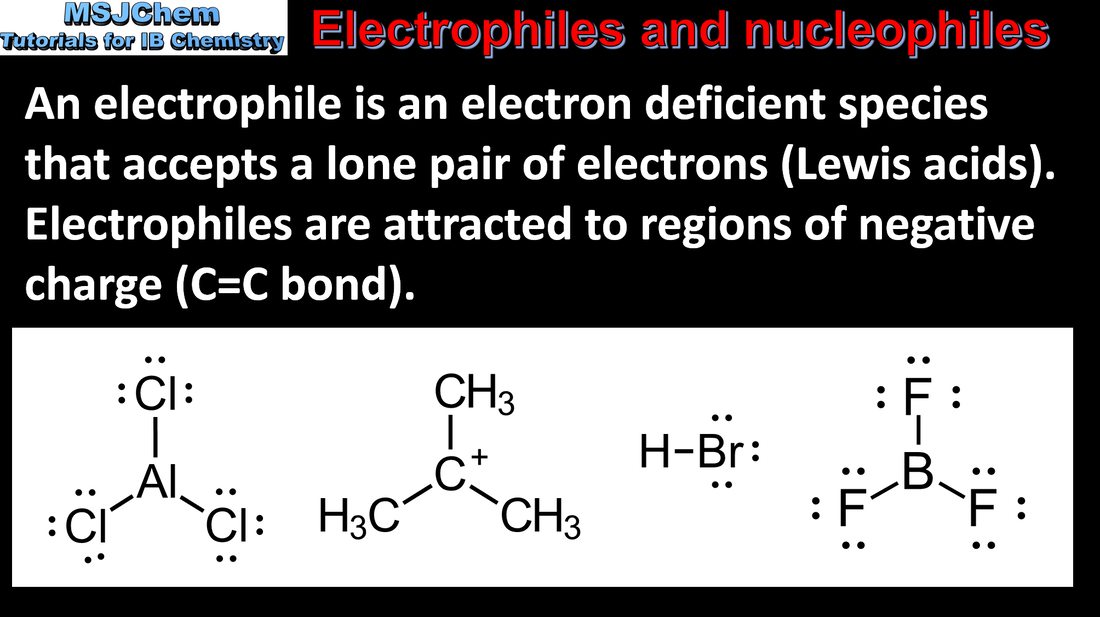
- An electrophile is a reactant that forms a bond to its reaction partner (the nucleophile) by accepting both bonding electrons from that reaction partner.
- Recognise electrophiles in chemical reactions.
- Both neutral and positively-charged species should be included.
Reactivity 3.4.5
Understandings:
Understandings:
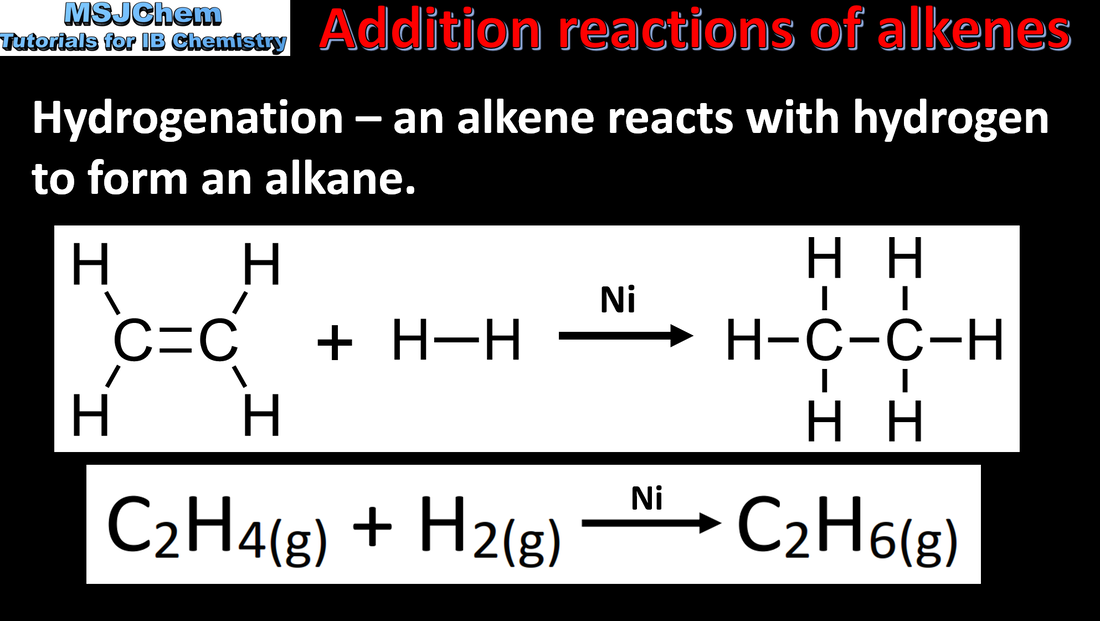
- Alkenes are susceptible to electrophilic attack because of the high electron density of the carbon–carbon double bond. These reactions lead to electrophilic addition.
- Deduce equations for the reactions of alkenes with water, halogens, and hydrogen halides.
- The mechanisms of these reactions will not be assessed at SL.
- Reactivity 3.3 Why is bromine water decolourized in the dark by alkenes but not by alkanes?
- Structure 2.4 Why are alkenes sometimes known as “starting molecules” in industry?