Structure 3.2 Functional groups: Classification of organic compounds (HL)
Structure 3.2.7
Understandings:
Understandings:
- Stereoisomers have the same constitution (atom identities, connectivities and bond multiplicities) but different spatial arrangements of atoms.
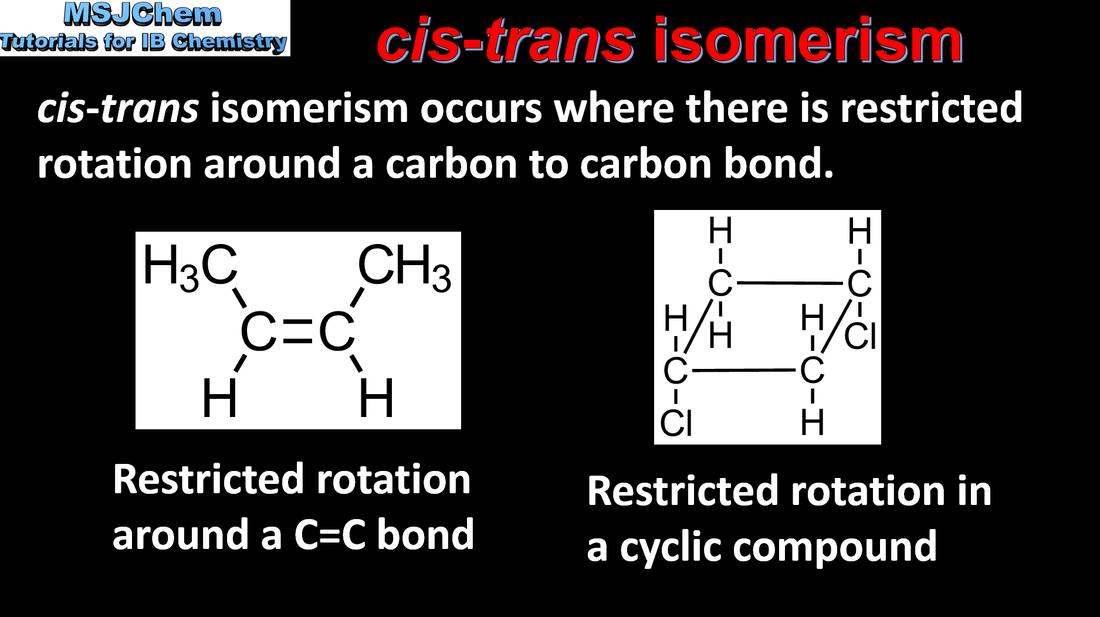
- Describe and explain the features that give rise to cis-trans isomerism; recognize it in non-cyclic alkenes and C3 and C4 cycloalkanes.
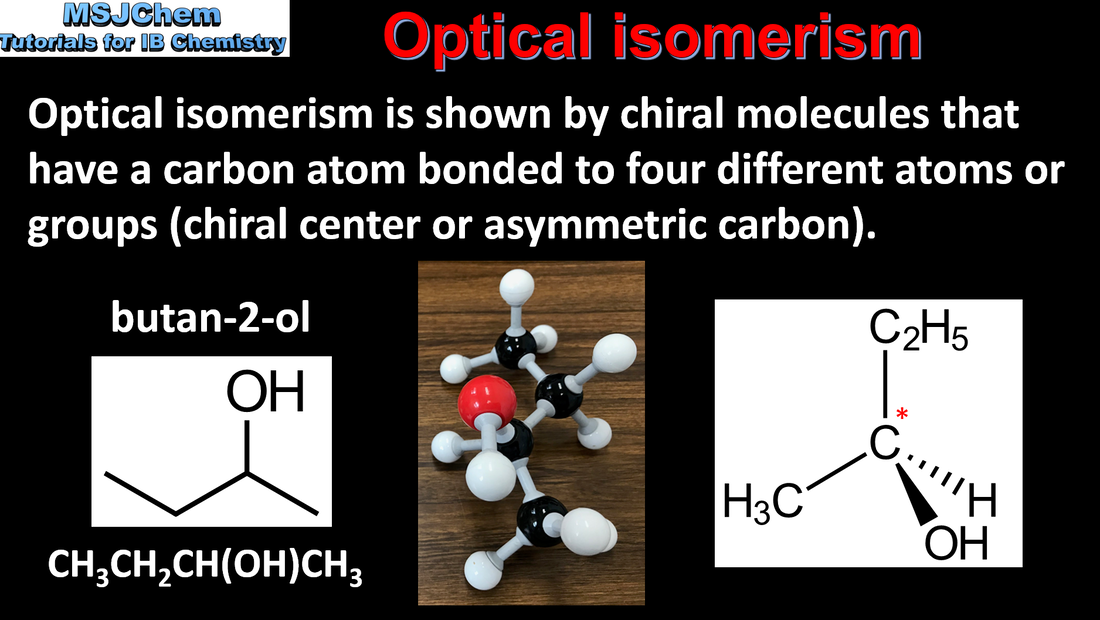
- Draw stereochemical formulas showing the tetrahedral arrangement around a chiral carbon.
- Describe and explain a chiral carbon atom giving rise to stereoisomers with different optical properties. Recognize a pair of enantiomers as non-superimposable mirror images from 3D modelling (real or virtual).
- Nomenclature using the E‐Z system will not be assessed.
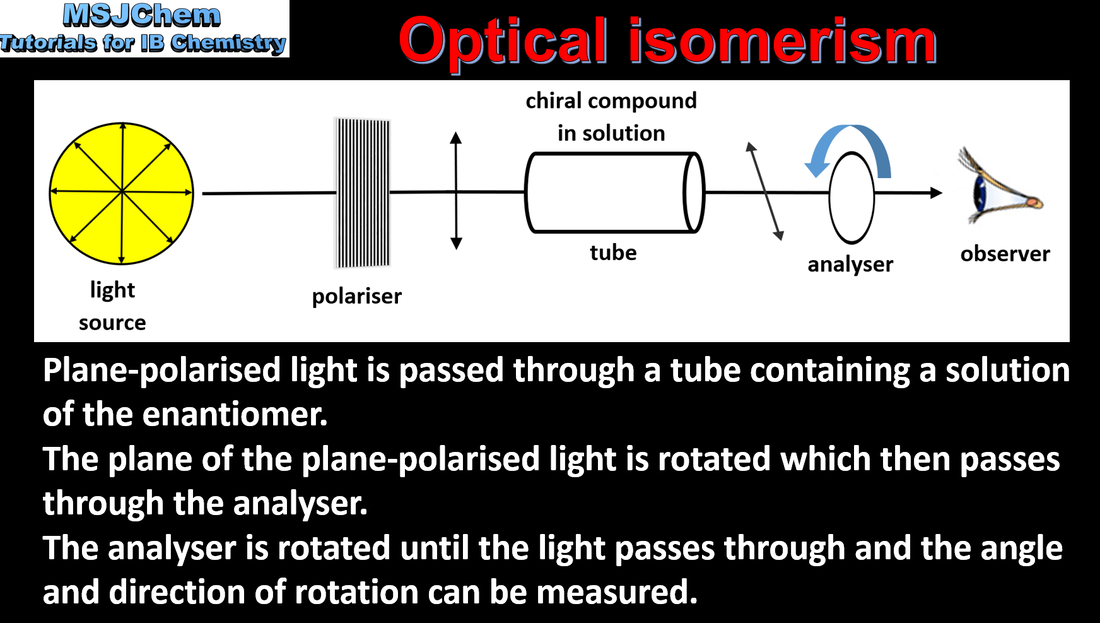
- The terms “chiral”, “optical activity”, “enantiomer” and “racemic” mixture should be understood.
- Different chemical properties of enantiomers can be limited to the fact that they behave differently in chiral environments.
- Wedge-dash type representations involving tapered bonds should be used for the representation of enantiomers.
Structure 3.2.8
Understandings:
Understandings:
- Mass spectrometry (MS) of organic compounds can cause fragmentation of molecules.
- Deduce information about the structural features of a compound from specific MS fragmentation patterns.
- Include reference to the molecular ion.
- Data on specific MS fragments are provided in the data booklet.
|
Video coming soon.
|
Structure 3.2.9
Understandings:
Understandings:
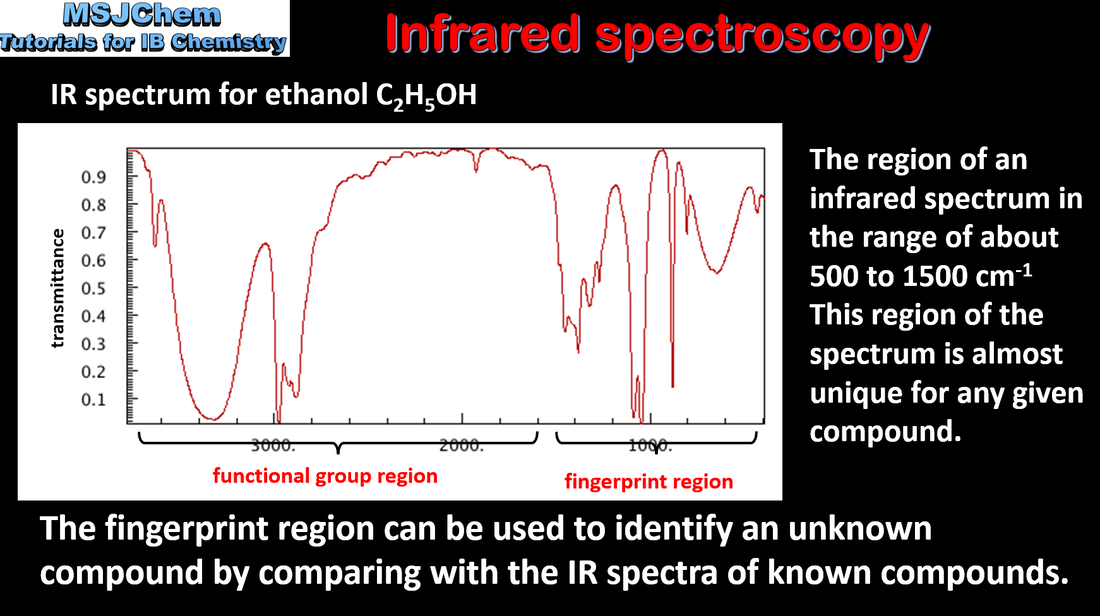
- Infrared (IR) spectra can be used to identify the type of bond present in a molecule.
- Interpret the functional group region of an IR spectrum, using a table of characteristic frequencies (wavenumber/cm–1).
- Include reference to the absorption of IR radiation by greenhouse gases.
- Data for interpretation of IR spectra are given in the data booklet.
- Reactivity 1.3 What properties of a greenhouse gas determine its “global warming potential”?
Structure 3.2.10
Understandings:
Understandings:
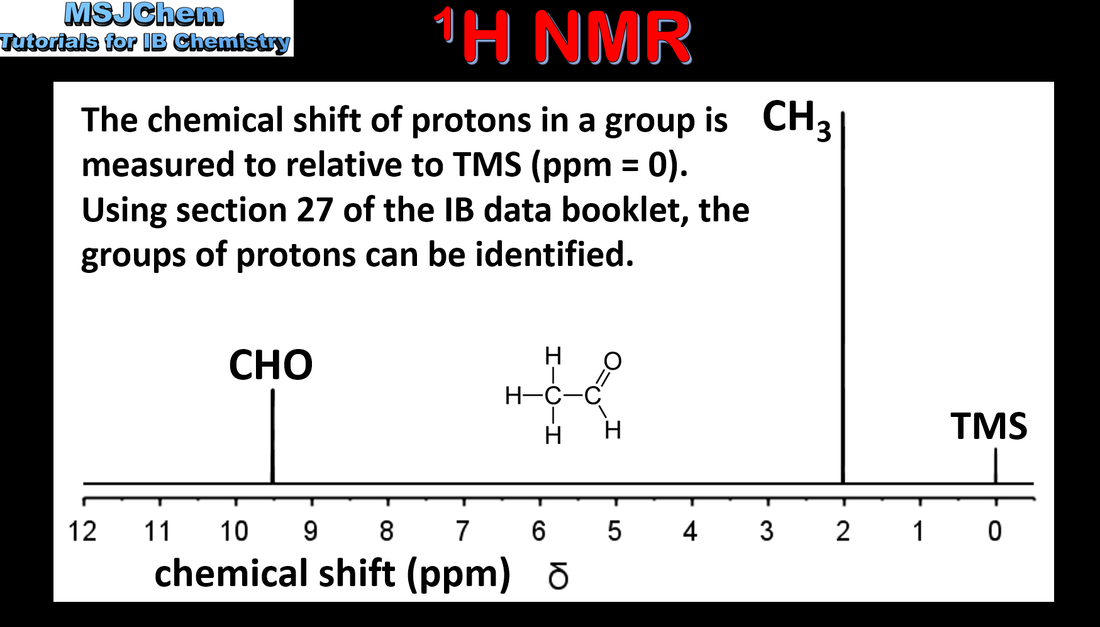
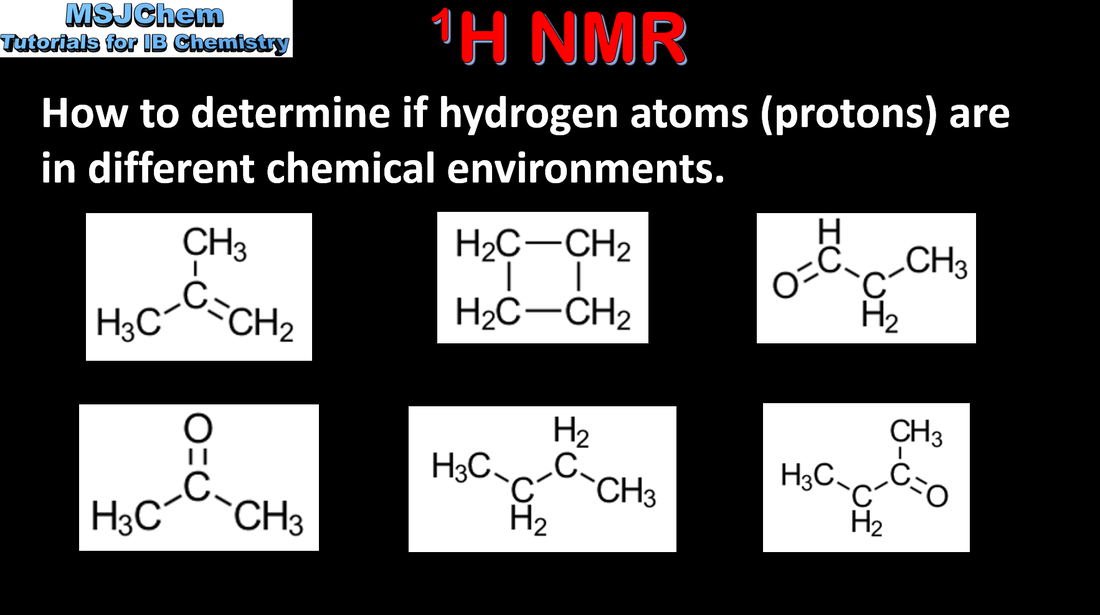
- Proton nuclear magnetic resonance spectroscopy (1H NMR) gives information on the different chemical environments of hydrogen atoms in a molecule.
- Interpret 1H NMR spectra to deduce the structures of organic molecules from the number of signals, the chemical shifts, and the relative areas under signals (integration traces).
Structure 3.2.11
Understandings:
Understandings:
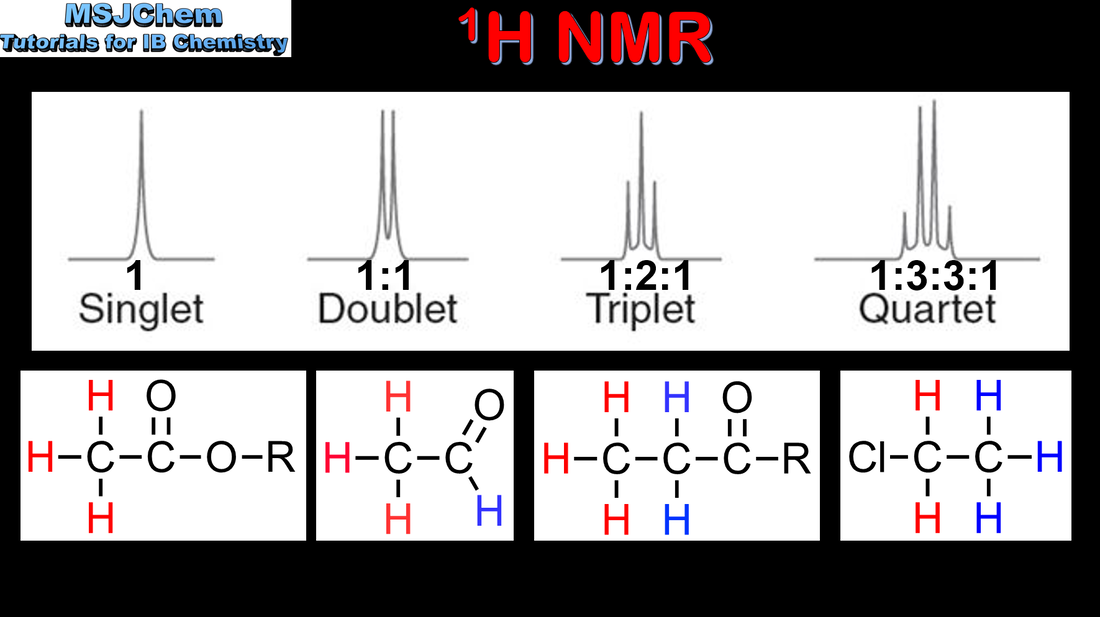
- Individual signals can be split into clusters of peaks.
- Interpret 1H NMR spectra from splitting patterns showing singlets, doublets, triplets and quartets to deduce greater structural detail.
- Data for interpretation of 1H NMR spectra are given in the data booklet.
Structure 3.2.12
Understandings:
Understandings:
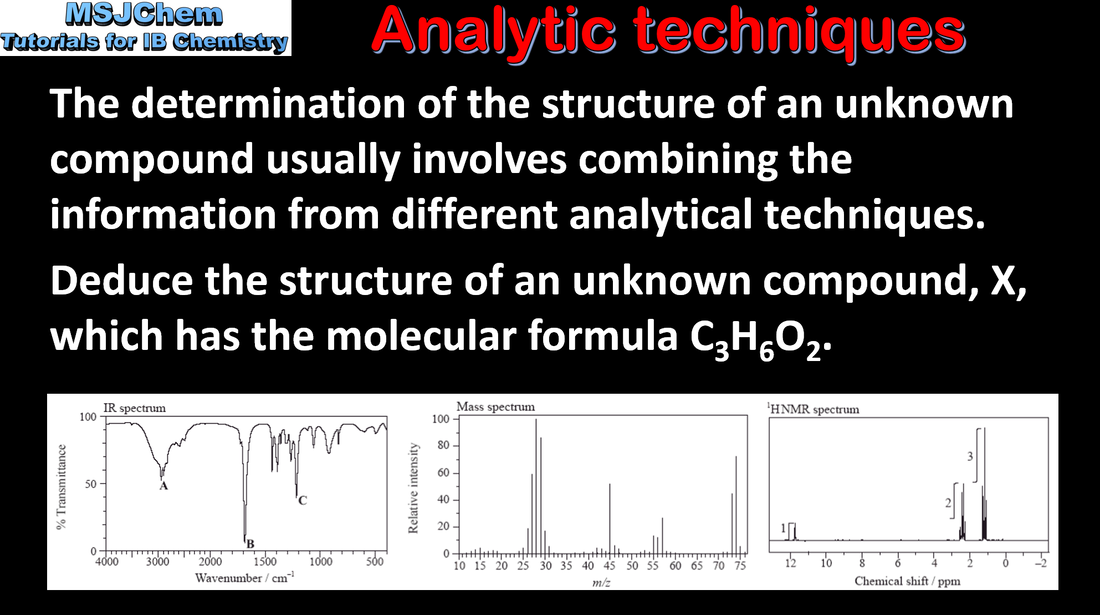
- Data from different techniques are often combined in structural analysis.
- Interpret a variety of data, including analytical spectra, to determine the structure of a molecule.