Reactivity 1.2 Energy cycles in reactions
Reactivity 1.2.1
Understandings:
Understandings:
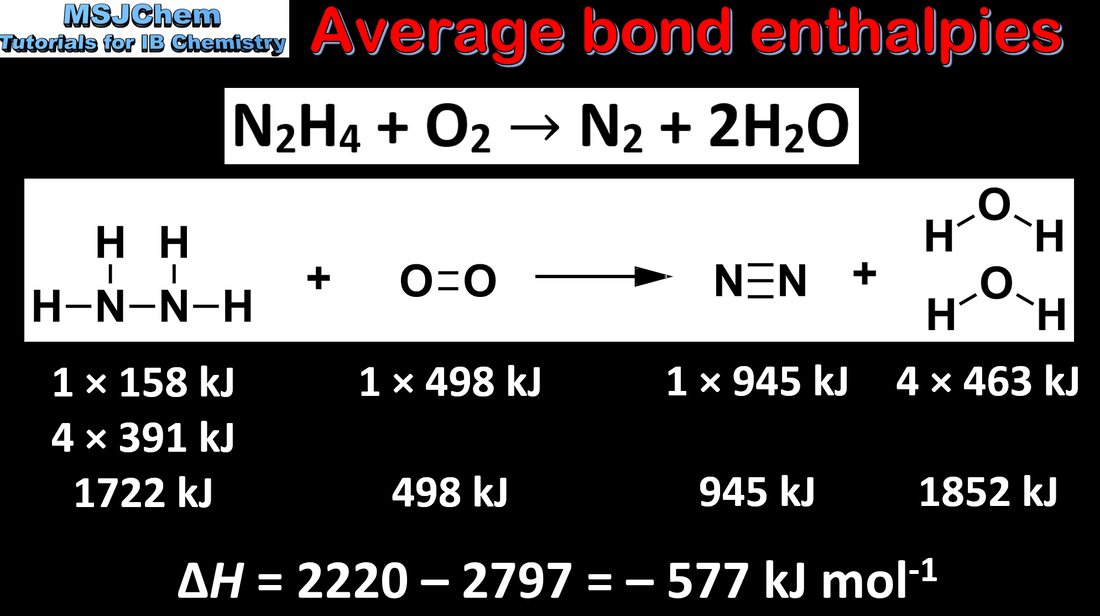
- Bond-breaking absorbs energy and bond-forming releases energy.
- Calculate the enthalpy change of a reaction from given average bond enthalpy data.
- Include explanation of why bond enthalpy data are average values and may differ from those measured experimentally.
- Average bond enthalpy values are given in the data booklet.
- Structure 2.2 How would you expect bond enthalpy data to relate to bond length and polarity?
- Reactivity 3.4 How does the strength of a carbon– halogen bond affect the rate of a nucleophilic substitution reaction?
Reactivity 1.2.2
Understandings:
Understandings:
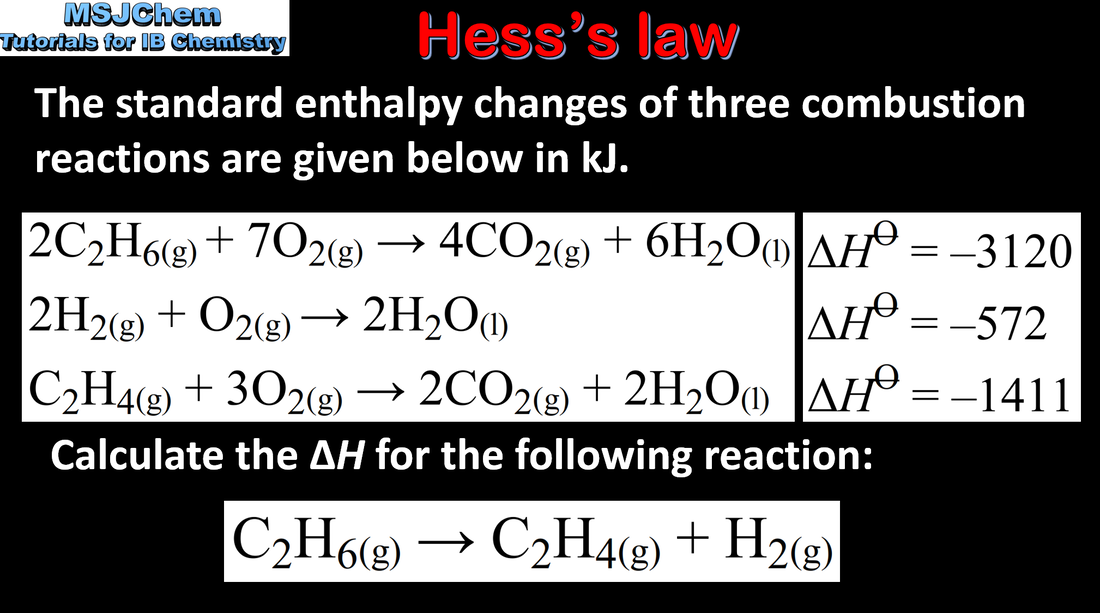
- Hess’s law states that the enthalpy change for a reaction is independent of the pathway between the initial and final states.
- Apply Hess’s law to calculate enthalpy changes in multistep reactions.