Reactivity 2.1 How much? The amount of chemical change
Reactivity 2.1.1 and 2.1.2
Understandings:
Understandings:
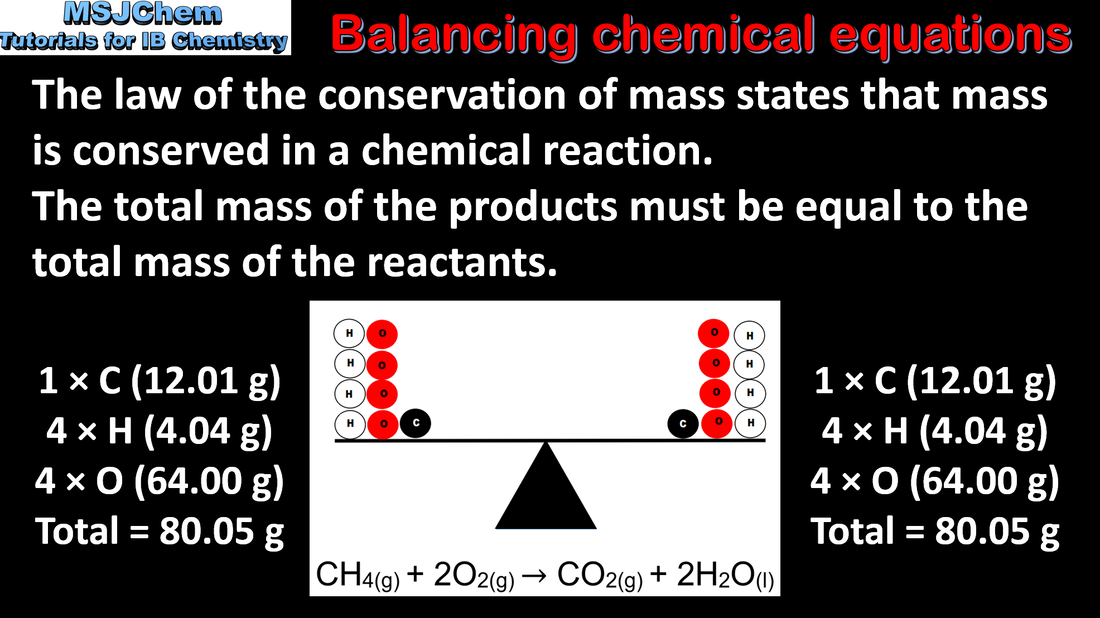
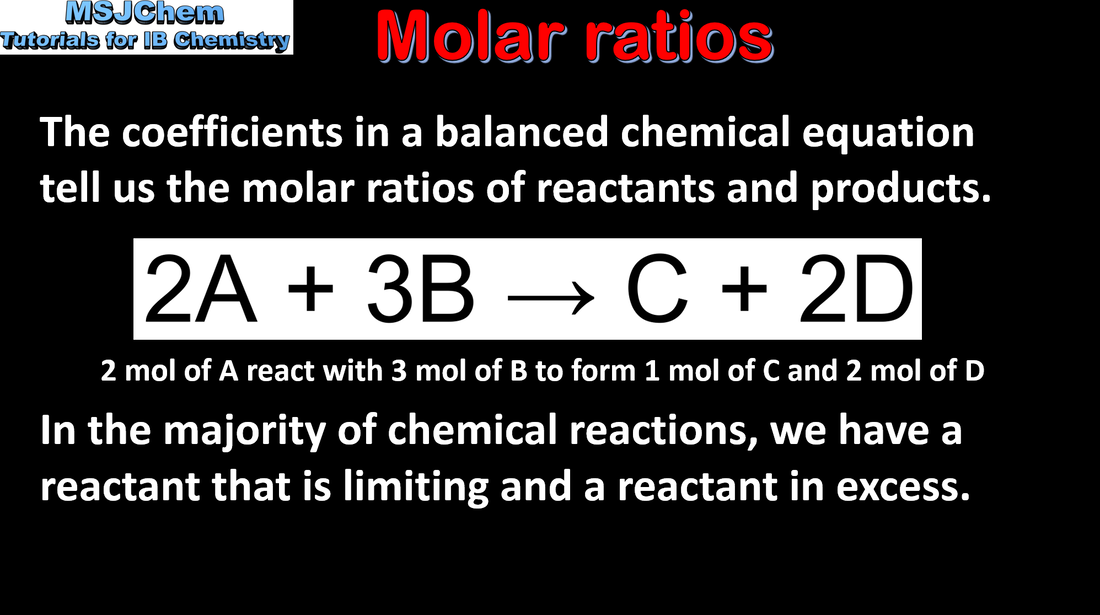
- Chemical equations show the ratio of reactants and products in a reaction (2.1.1).
- The mole ratio of an equation can be used to determine:
- the masses and/or volumes of reactants and products
the concentrations of reactants and products for reactions occurring in solution (2.1.2).
- Deduce chemical equations when reactants and products are specified (2.1.1).
- Calculate reacting masses and/or volumes and concentrations of reactants and products (2.1.2).
- Include the use of state symbols in chemical equations.
- Avogadro’s law and definitions of molar concentration are covered in Structure 1.4.
- The values for Ar given in the data booklet to two decimal places should be used in calculations.
- Reactivity 3.2 When is it useful to use half- equations?
- Structure 1.5 How does the molar volume of a gas vary with changes in temperature and pressure?
Reactivity 2.1.3
Understandings:
Understandings:
- The limiting reactant determines the theoretical yield.
- Identify the limiting and excess reactants from given data.
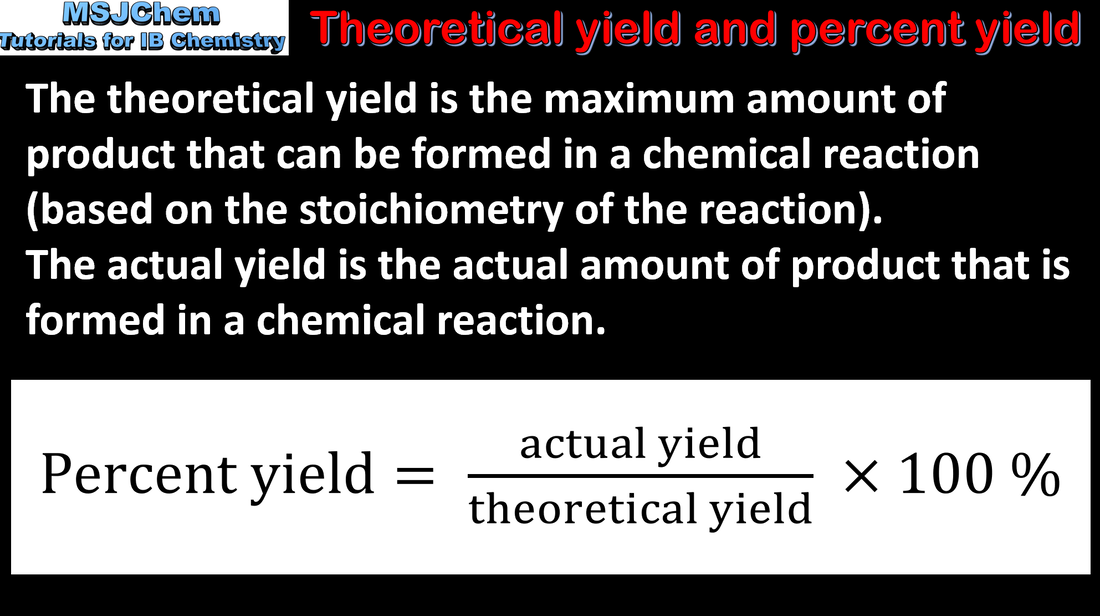
- Distinguish between the theoretical yield and the experimental yield.
Reactivity 2.1.4
Understandings:
Understandings:
- The percentage yield is calculated from the ratio of experimental yield to theoretical yield.
- Solve problems involving reacting quantities, limiting and excess reactants, theoretical, experimental and percentage yields.
Reactivity 2.1.5
Understandings:
Understandings:
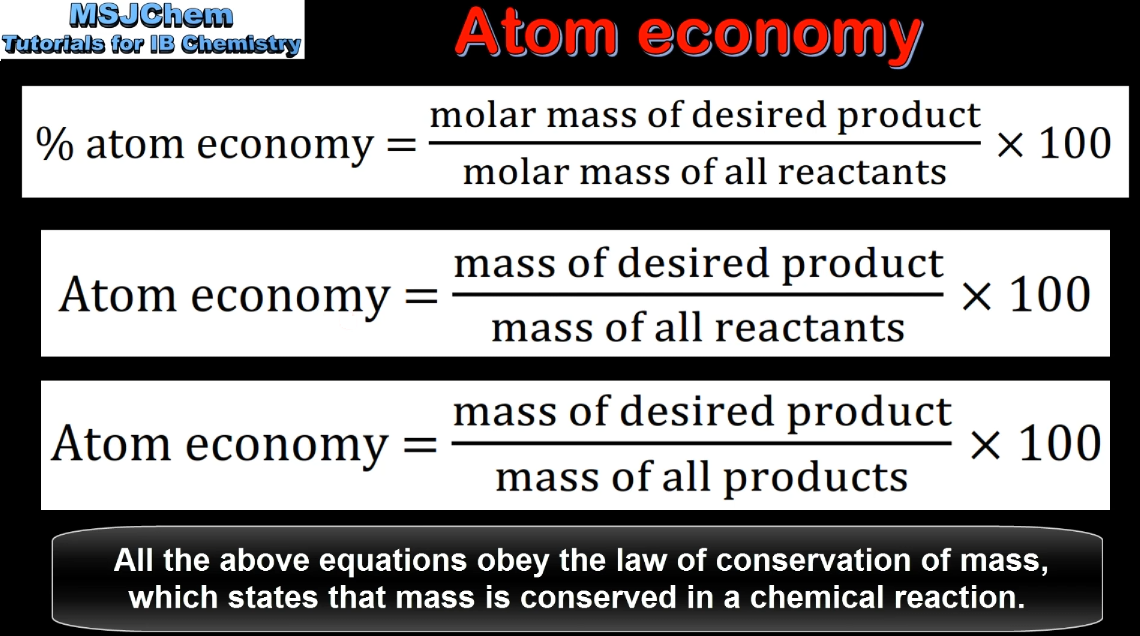
- The atom economy is a measure of efficiency in green chemistry.
- Calculate the atom economy from the stoichiometry of a reaction.
- Include discussion of the inverse relationship between atom economy and wastage in industrial processes.
- The equation for calculation of the atom economy is given in the data booklet.
- Structure 2.4, Reactivity 2.2 The atom economy and the percentage yield both give important information about the “efficiency” of a chemical process. What other factors should be considered in this assessment?