Structure 1.2 The nuclear atom
Structure 1.2.1
Understandings:
Understandings:
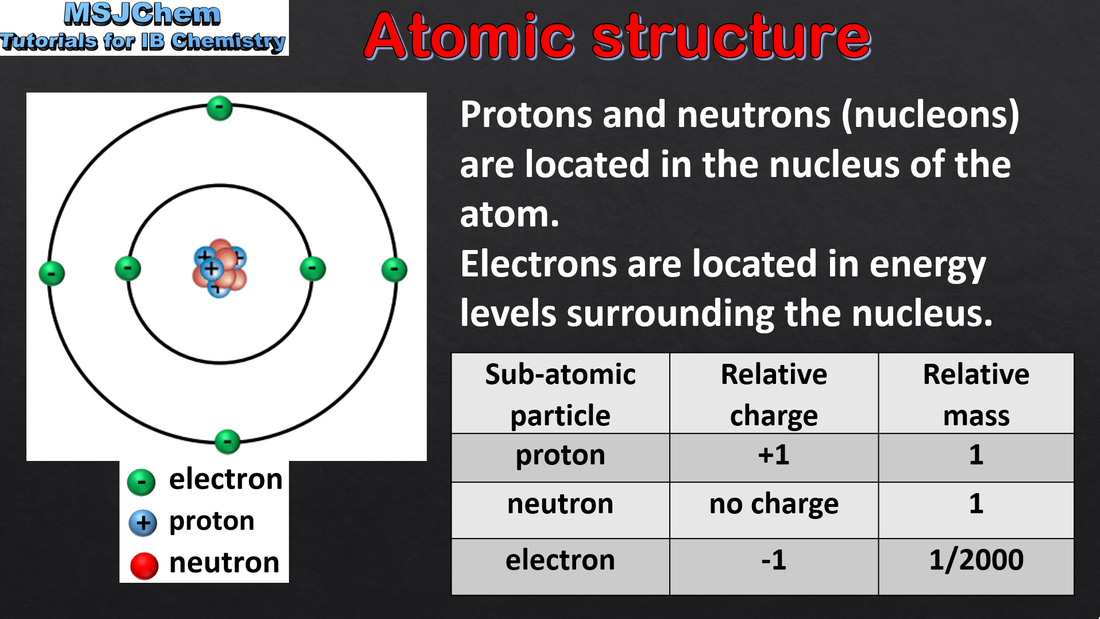
- Atoms contain a positively charged, dense nucleus composed of protons and neutrons (nucleons). Negatively charged electrons occupy the space outside the nucleus.
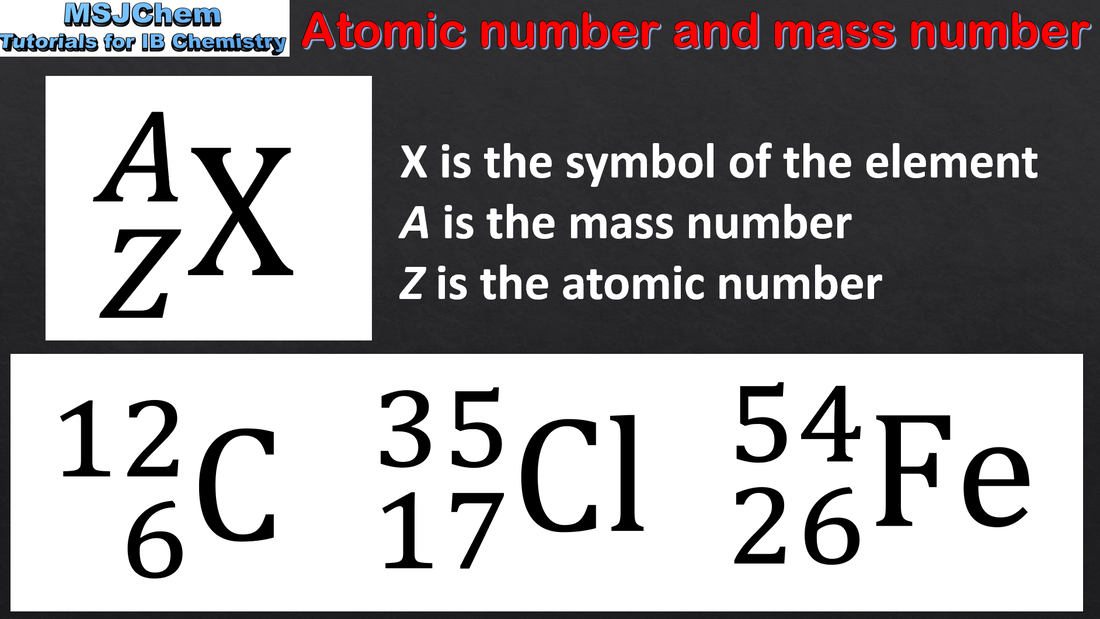
- Use the nuclear symbol to deduce the number of protons, neutrons and electrons in atoms and ions.
- Relative masses and charges of the subatomic particles should be known; actual values are given in the data booklet. The mass of the electron can be considered negligible.
Structure 1.2.2
Understandings:
Understandings:
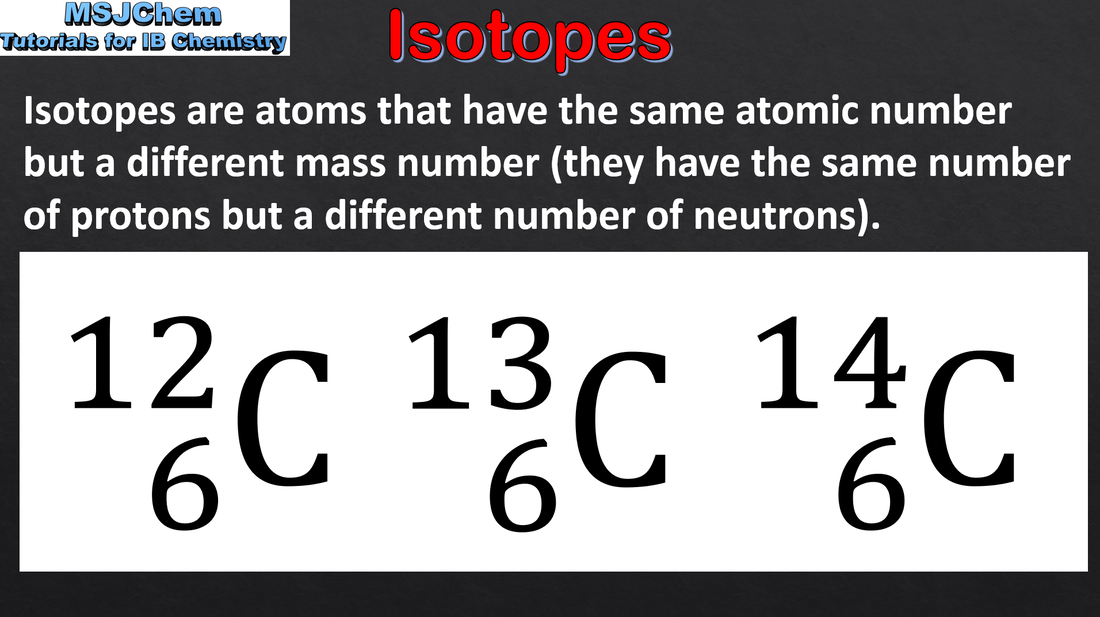
- Isotopes are atoms of the same element with different numbers of neutrons.
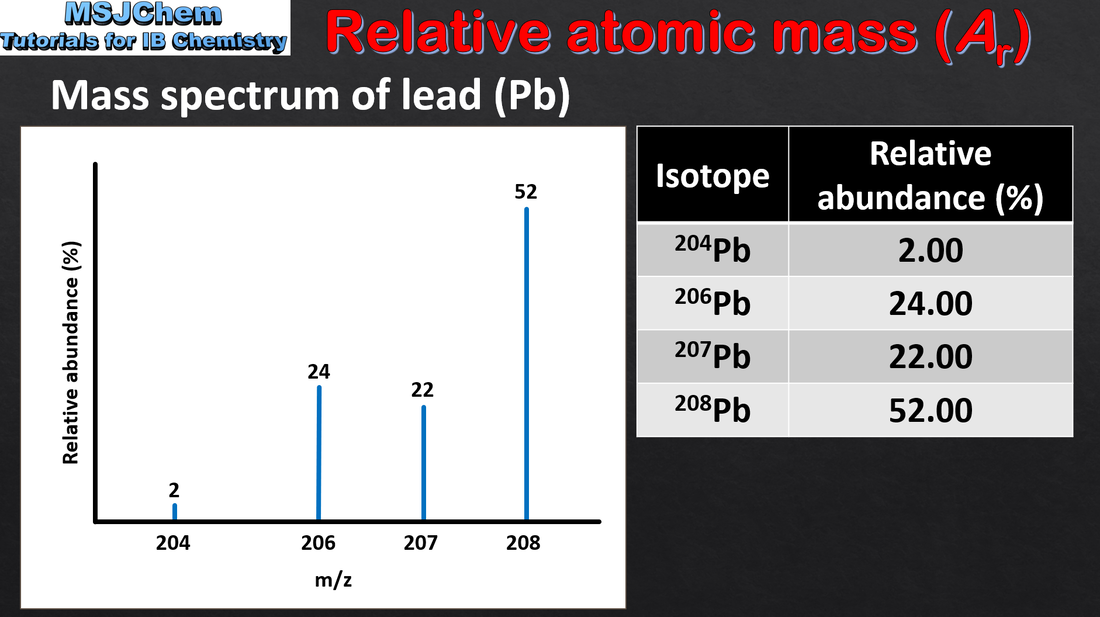
- Perform calculations involving non-integer relative atomic masses and abundance of isotopes from given data.
- Differences in the physical properties of isotopes should be understood.