Reactivity 3.2 Electron transfer reactions
Reactivity 3.2.1
Understandings:
Understandings:
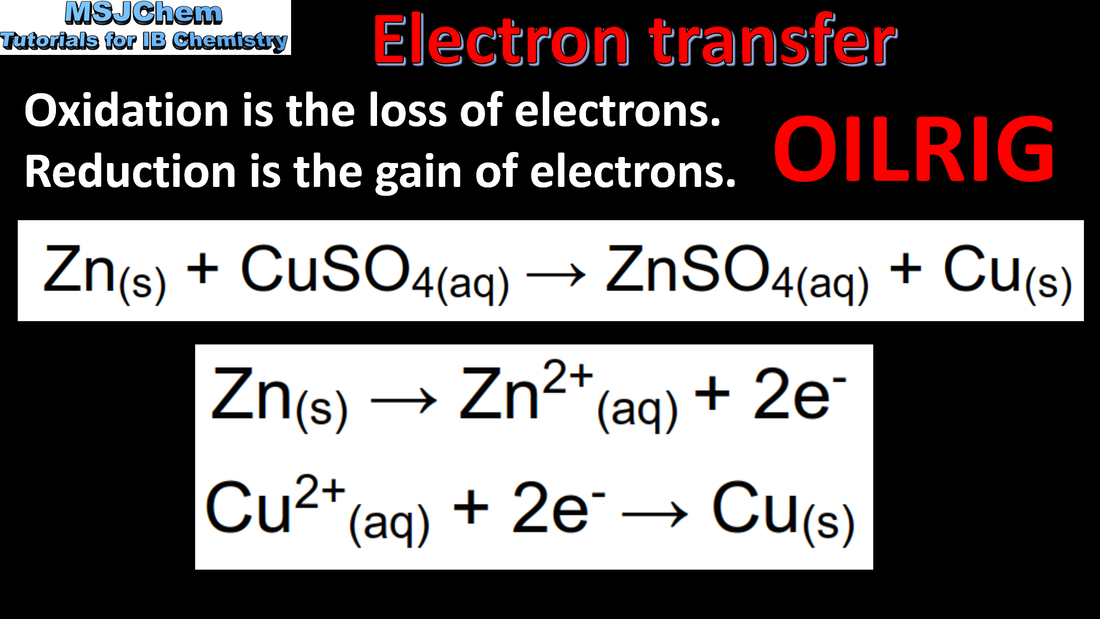
- Oxidation and reduction can be described in terms of electron transfer, change in oxidation state, oxygen gain/loss or hydrogen loss/gain.
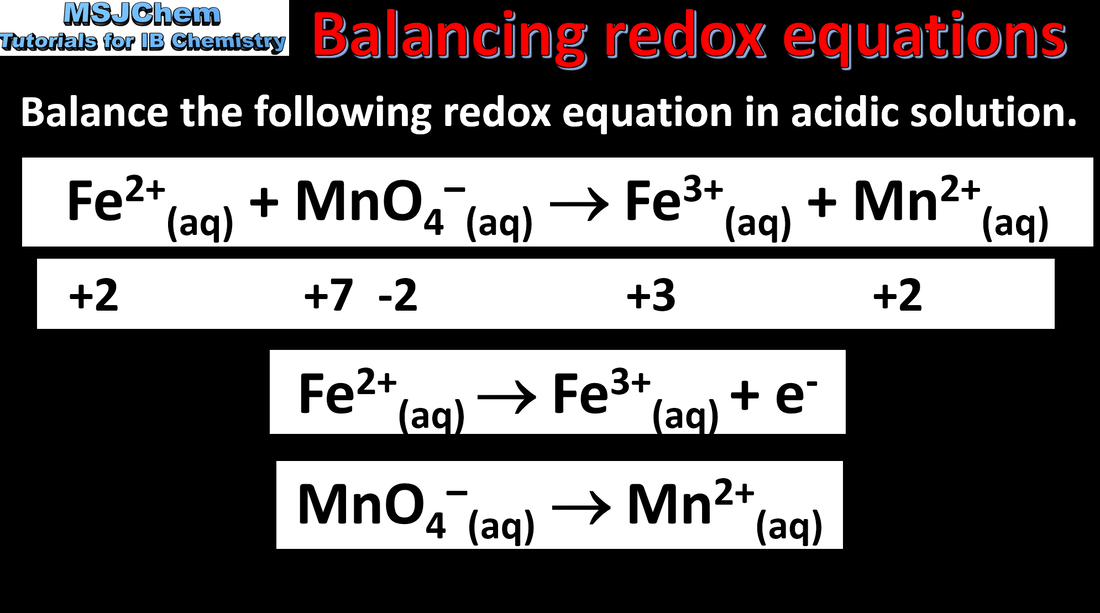
- Deduce oxidation states of an atom in a compound or an ion.
- Identify the oxidized and reduced species and the oxidizing and reducing agents in a chemical reaction.
- Include examples to illustrate the variable oxidation states of transition element ions and of most main group non-metals.
- Include the use of oxidation numbers in the naming of compounds.
- Structure 3.1 What are the advantages and limitations of using oxidation states to track redox changes?
- Structure 2.3 The surface oxidation of metals is often known as corrosion. What are some of the consequences of this process?
Reactivity 3.2.2
Understandings:
Understandings:
- Half-equations separate the processes of oxidation and reduction, showing the loss or gain of electrons.
- Deduce redox half-equations and equations in acidic or neutral solutions.
Reactivity 3.2.3
Understandings:
Understandings:
- The relative ease of oxidation and reduction of an element in a group can be predicted from its position in the periodic table.
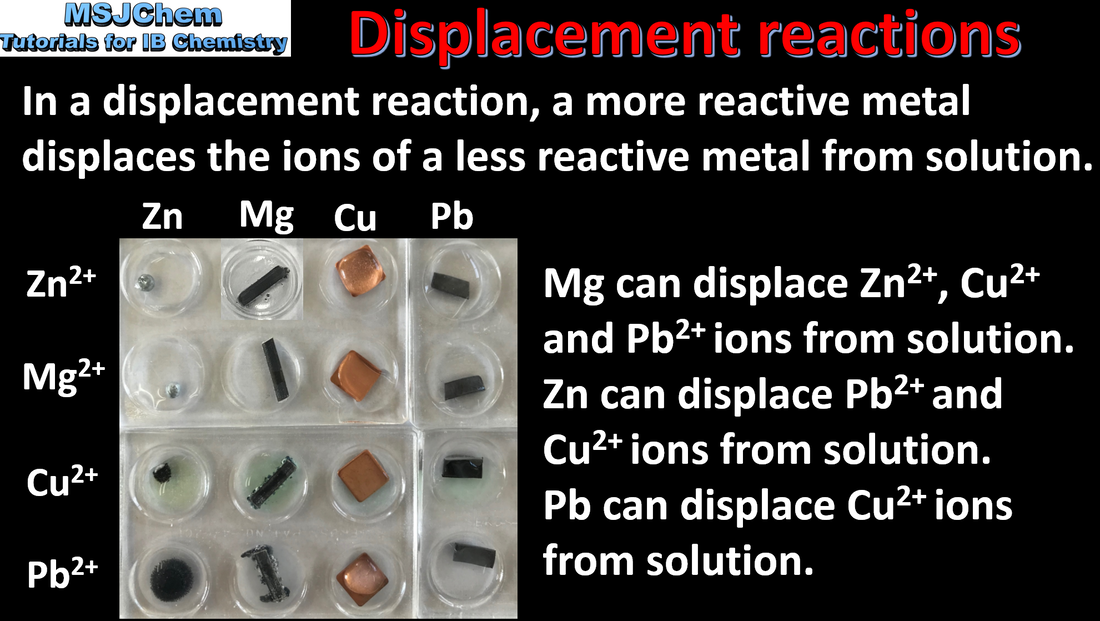
- The reactions between metals and aqueous metal ions demonstrate the relative ease of oxidation of different metals.
- Predict the relative ease of oxidation of metals.
- Predict the relative ease of reduction of halogens.
- Interpret data regarding metal and metal ion reactions.
- The relative reactivity of metals observed in metal/ metal ion displacement reactions does not need to be learned; appropriate data will be supplied in examination questions.
- Structure 3.1 Why does metal reactivity increase, and non-metal reactivity decrease, down the main groups of the periodic table?
Reactivity 3.2.4
Understandings:
Understandings:
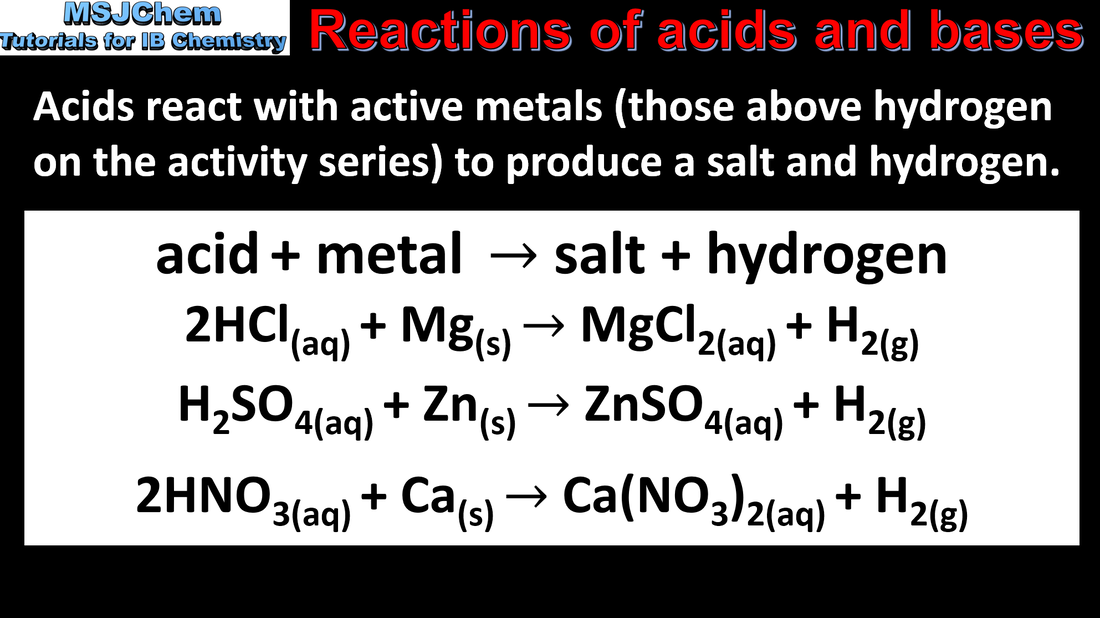
- Acids react with reactive metals to release hydrogen.
- Deduce equations for reactions of reactive metals with dilute HCl and H2SO4.
Reactivity 3.2.5
Understandings:
Understandings:
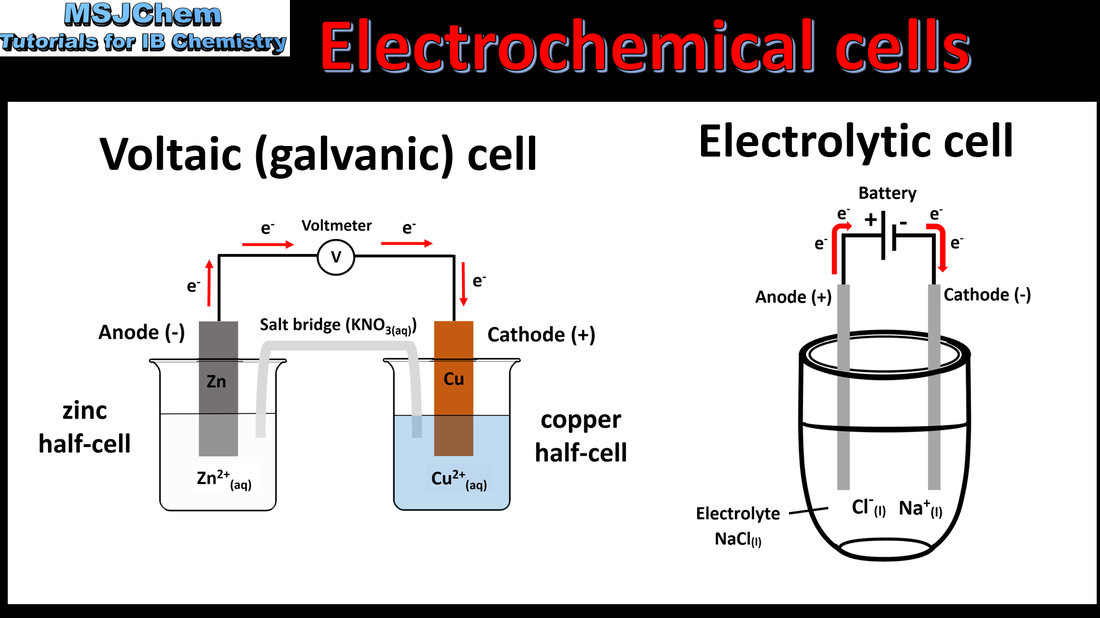
- Oxidation occurs at the anode and reduction occurs at the cathode in electrochemical cells.
- Label electrodes as anode and cathode, and identify their signs/polarities in voltaic cells and electrolytic cells, based on the type of reaction occurring at the electrode.
Reactivity 3.2.6
Understandings:
Understandings:
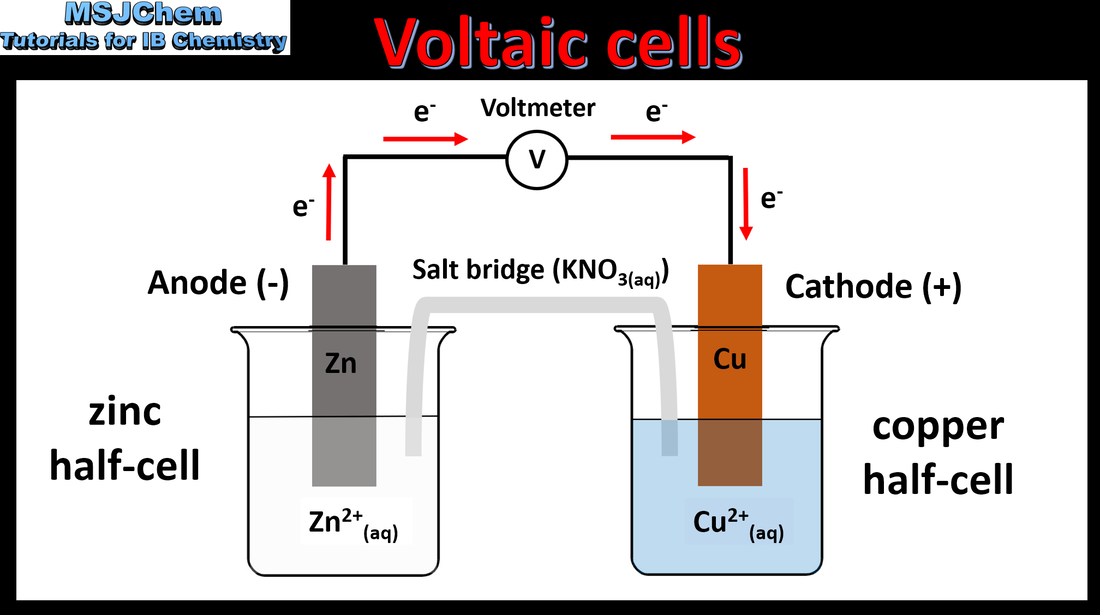
- A primary (voltaic) cell is an electrochemical cell that converts energy from spontaneous redox reactions to electrical energy.
- Explain the direction of electron flow from anode to cathode in the external circuit, and ion movement across the salt bridge.
- Construction of primary cells should include: half- cells containing metal/metal ion, anode, cathode, electric circuit, salt bridge.
- Reactivity 1.3 Electrical energy can be derived from the combustion of fossil fuels or from electrochemical reactions. What are the similarities and differences in these reactions?
Reactivity 3.2.7
Understandings:
Understandings:
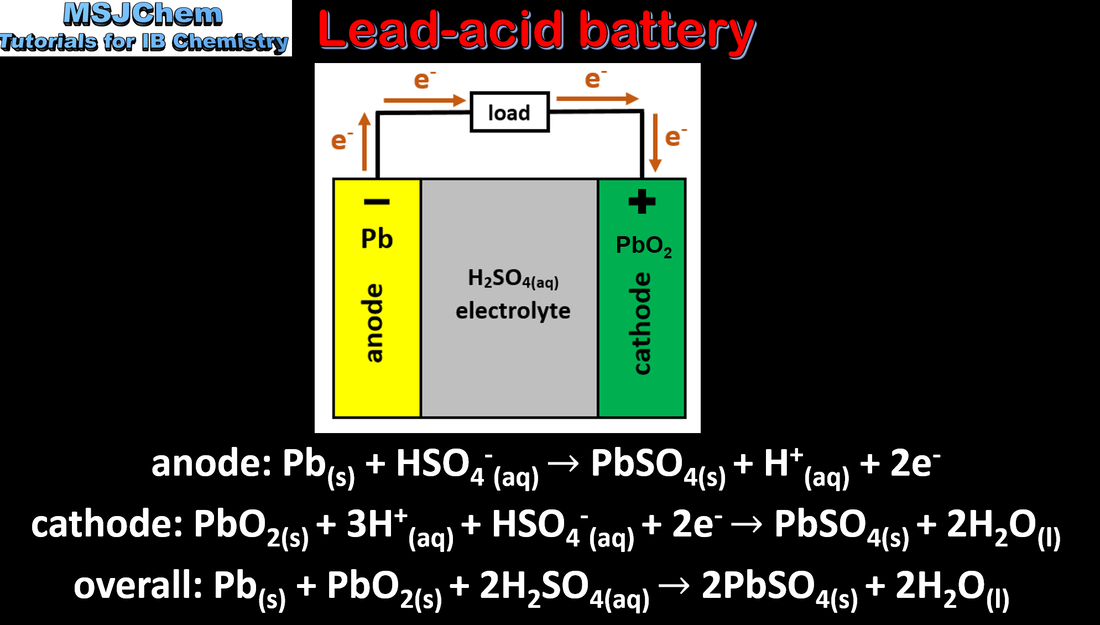
- Secondary (rechargeable) cells involve redox reactions that can be reversed using electrical energy.
- Deduce the reactions of the charging process from given electrode reactions for discharge, and vice versa.
- Include discussion of advantages and disadvantages of fuel cells, primary cells and secondary cells.
- Reactivity 2.3 Secondary cells rely on electrode reactions that are reversible. What are the common features of these reactions?
Reactivity 3.2.8
Understandings:
Understandings:
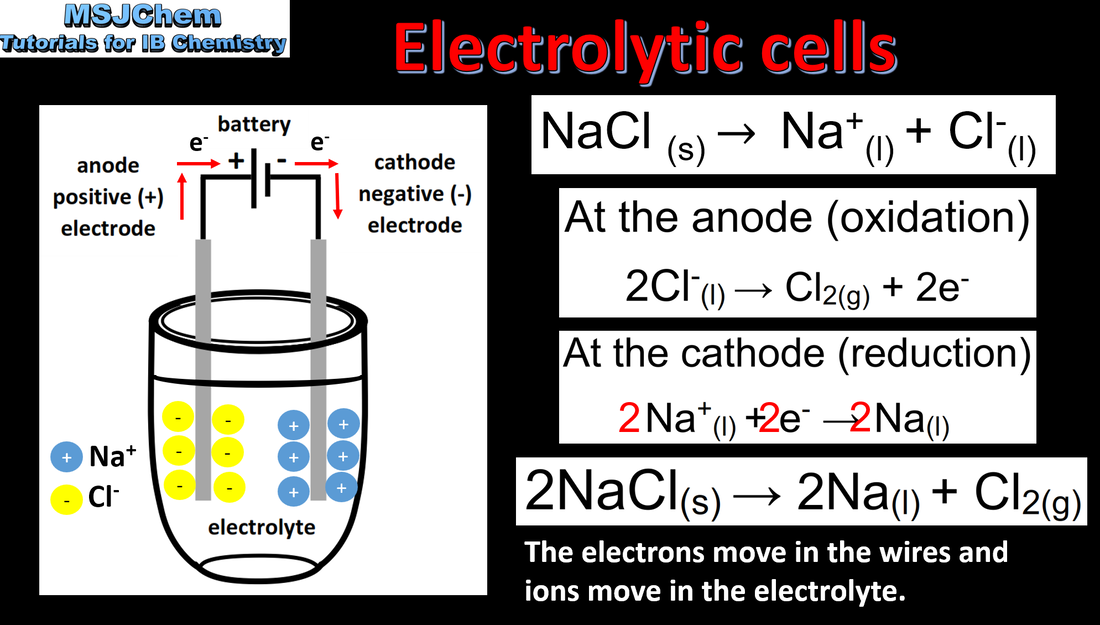
- An electrolytic cell is an electrochemical cell that converts electrical energy to chemical energy by bringing about non-spontaneous reactions.
- Explain how current is conducted in an electrolytic cell.
Deduce the products of the electrolysis of a molten salt.
- Construction of electrolytic cells should include: DC power source connected to anode and cathode, electrolyte.
- Structure 2.1 Under what conditions can ionic compounds act as electrolytes?
Reactivity 3.2.9
Understandings:
Understandings:
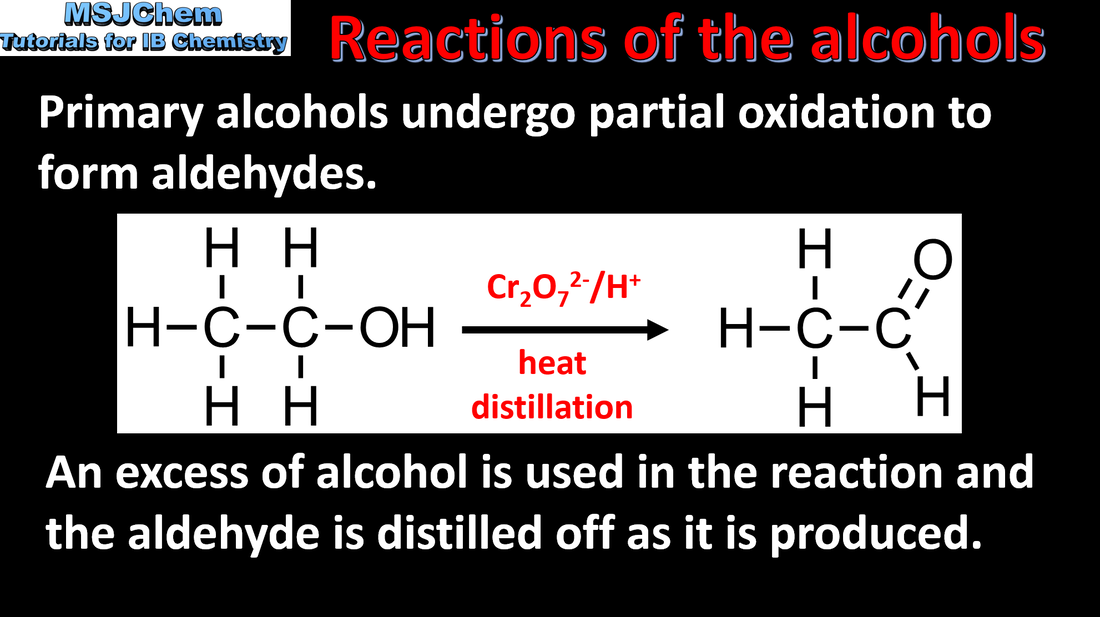
- Functional groups in organic compounds may undergo oxidation.
- Deduce equations to show changes in the functional groups during oxidation of primary and secondary alcohols, including the two-step reaction in the oxidation of primary alcohols.
- Include explanation of the experimental set-up for distillation and reflux.
- Include the fact that tertiary alcohols are not oxidized under similar conditions.
- Names and formulas of specific oxidizing agents and the mechanism will not be assessed.
- Structure 3.2 How does the nature of the functional group in a molecule affect its physical properties, such as boiling point?
- Reactivity 1.3 What is the difference between combustion and oxidation of an alcohol?
Reactivity 3.2.10
Understandings:
Understandings:
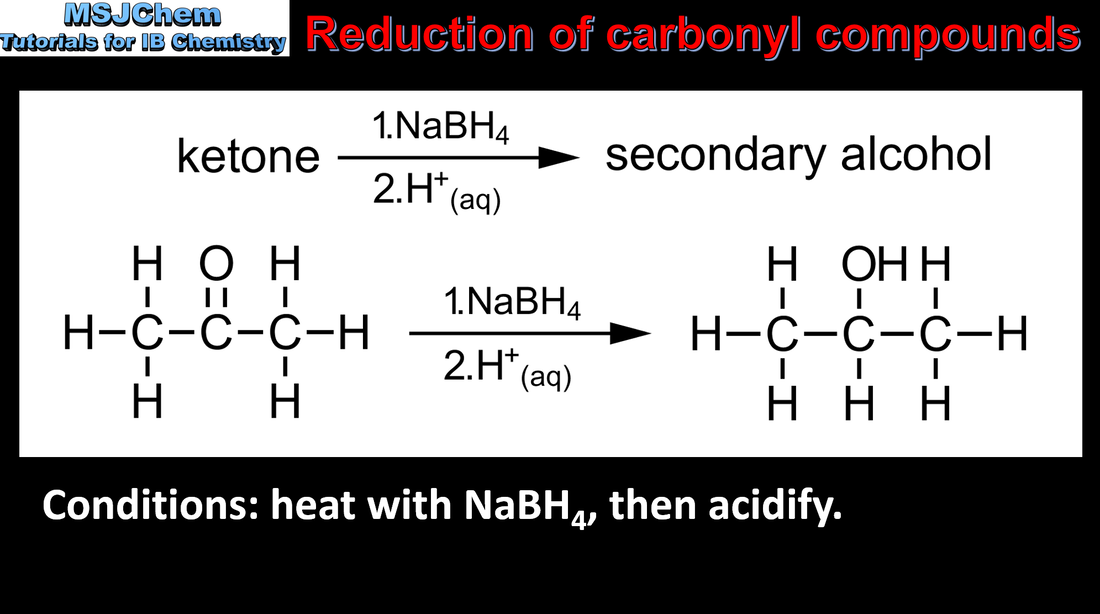
- Functional groups in organic compounds may undergo reduction.
- Deduce equations to show reduction of carboxylic acids to primary alcohols via the aldehyde, and reduction of ketones to secondary alcohols.
- Include the role of hydride ions in the reduction reaction.
- Names and formulas of specific reducing agents and the mechanisms will not be assessed.
- Structure 3.1 How can oxidation states be used to show that the following molecules are given in increasing order of oxidation: CH4, CH3OH, HCHO, HCOOH, CO2?
Reactivity 3.2.11
Understandings:
Understandings:
- Reduction of unsaturated compounds by the addition of hydrogen lowers the degree of unsaturation.
- Deduce the products of the reactions of hydrogen with alkenes and alkynes.
- Reactivity 3.4 Why are some reactions of alkenes classified as reduction reactions while others are classified as electrophilic addition reactions?
|
Video coming soon
|