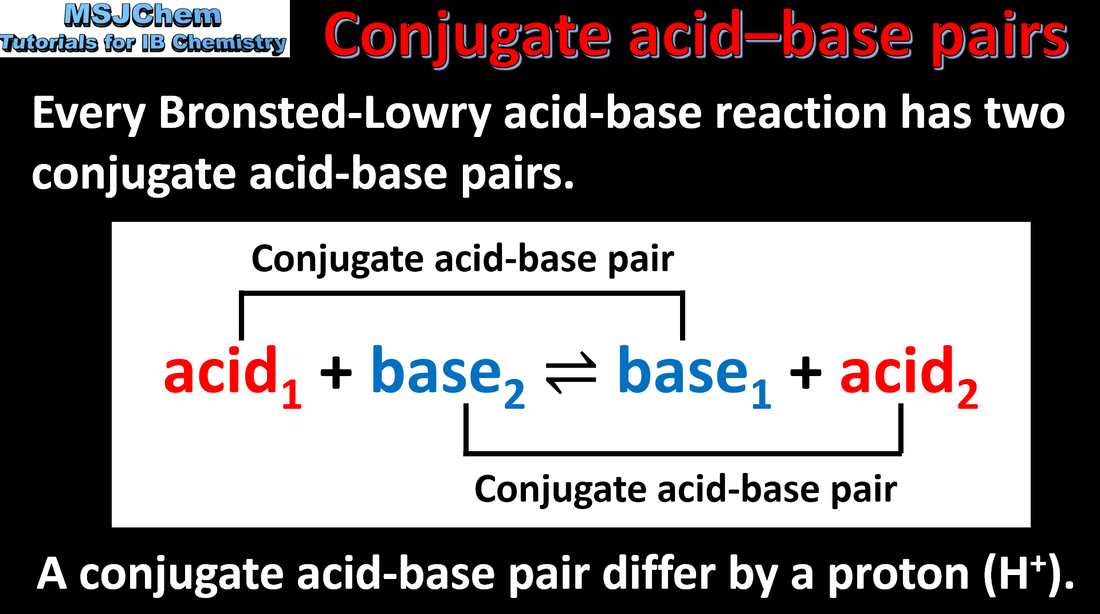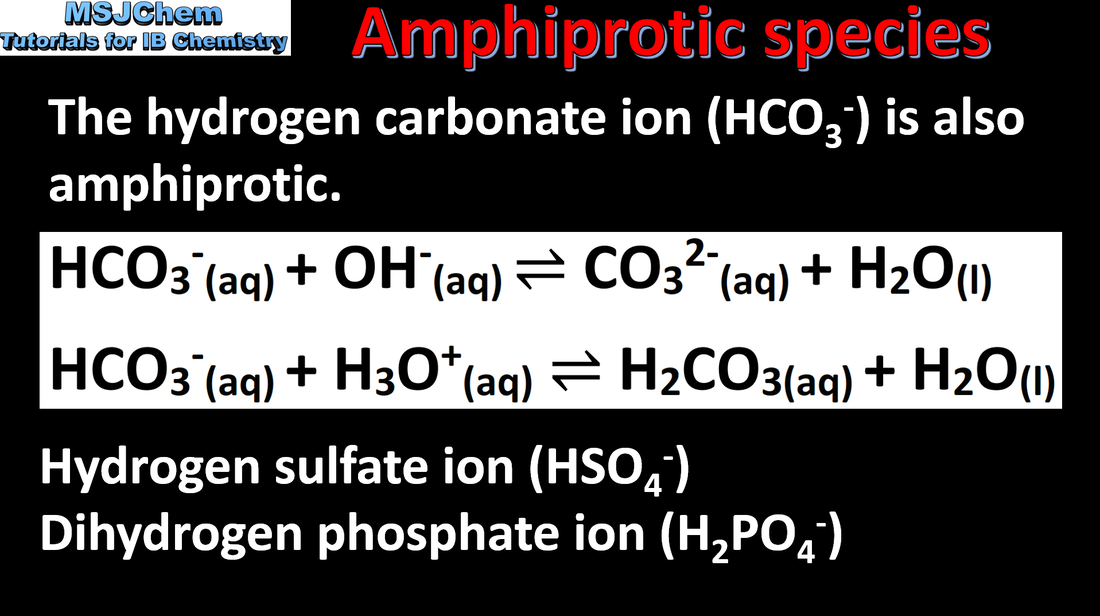Help support my work by joining the Member's Area or by becoming a Patron.
Topic 8 Acids and bases
YouTube playlist
YouTube playlist
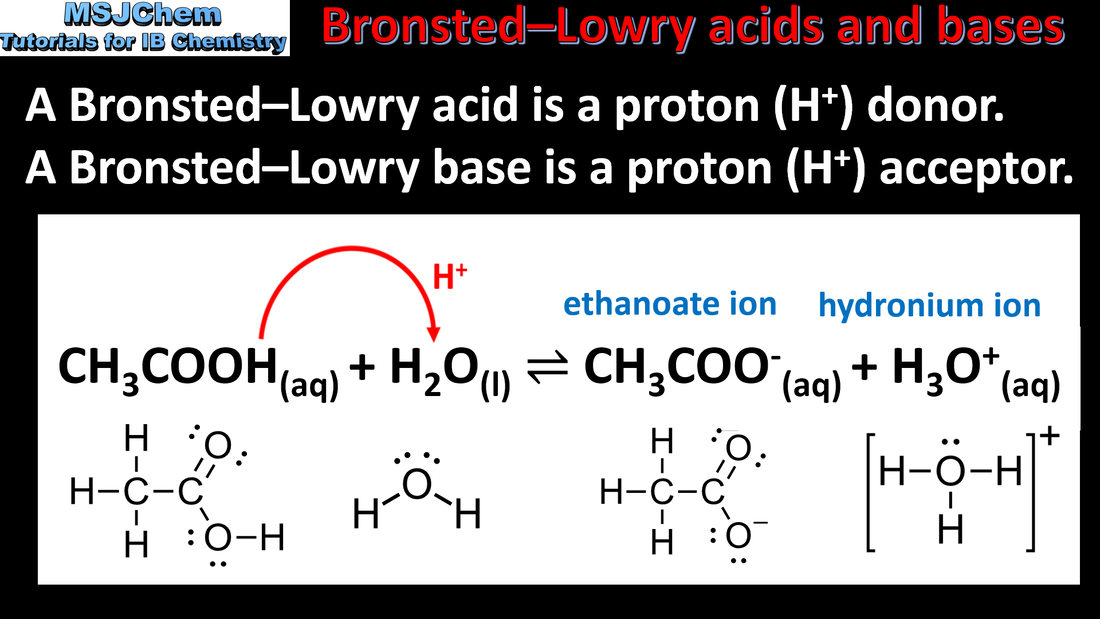
8.1 Brønsted–Lowry acids and bases / conjugate acid-base pairs
8.1 Conjugate acid-base pairs
8.1 Amphiprotic species
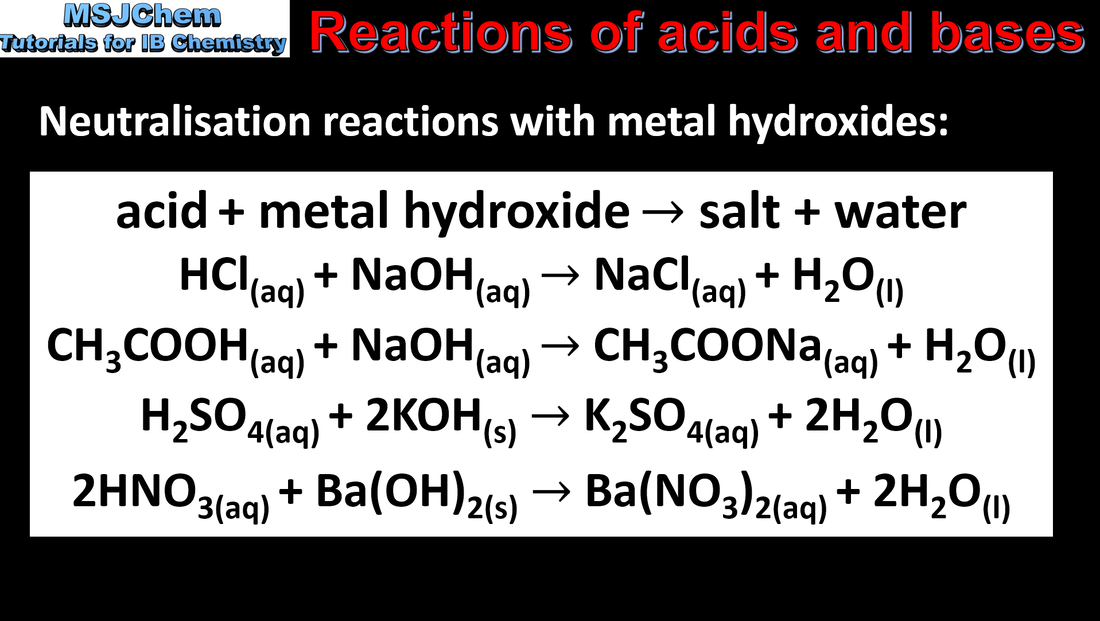
8.2 Reactions of acids and bases
|
Understandings:
Most acids have observable characteristic chemical reactions with reactive metals, metal oxides, metal hydroxides, hydrogen carbonates and carbonates. Salt and water are produced in exothermic neutralization reactions. Applications and skills: Balancing chemical equations for the reaction of acids. Identification of the acid and base needed to make different salts. Guidance: Bases which are not hydroxides, such as ammonia, soluble carbonates and hydrogen carbonates should be covered. |
8.2 Thermometric titration
|
|
This video covers how to calculate the concentration of a solution using a thermometric titration.
|
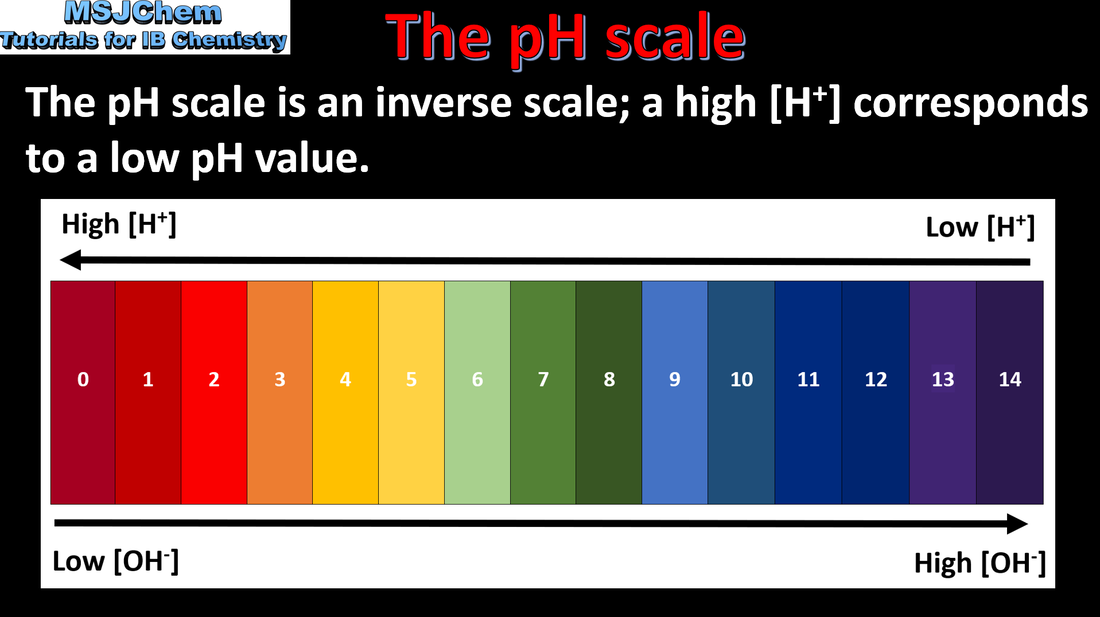
8.3 The pH scale
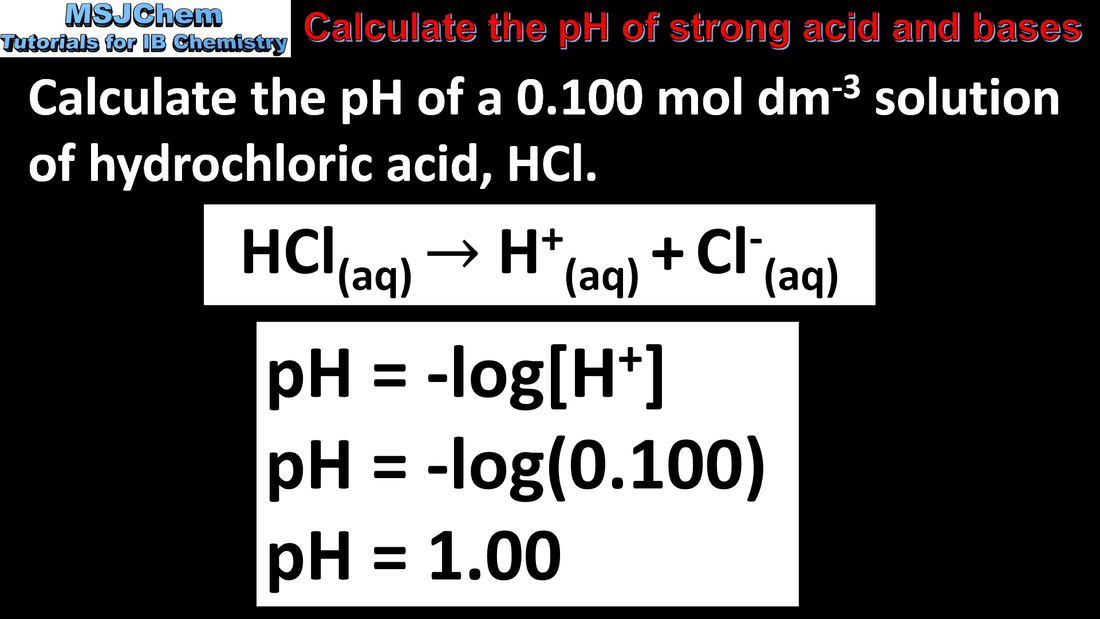
8.3 Calculating the pH of strong acids
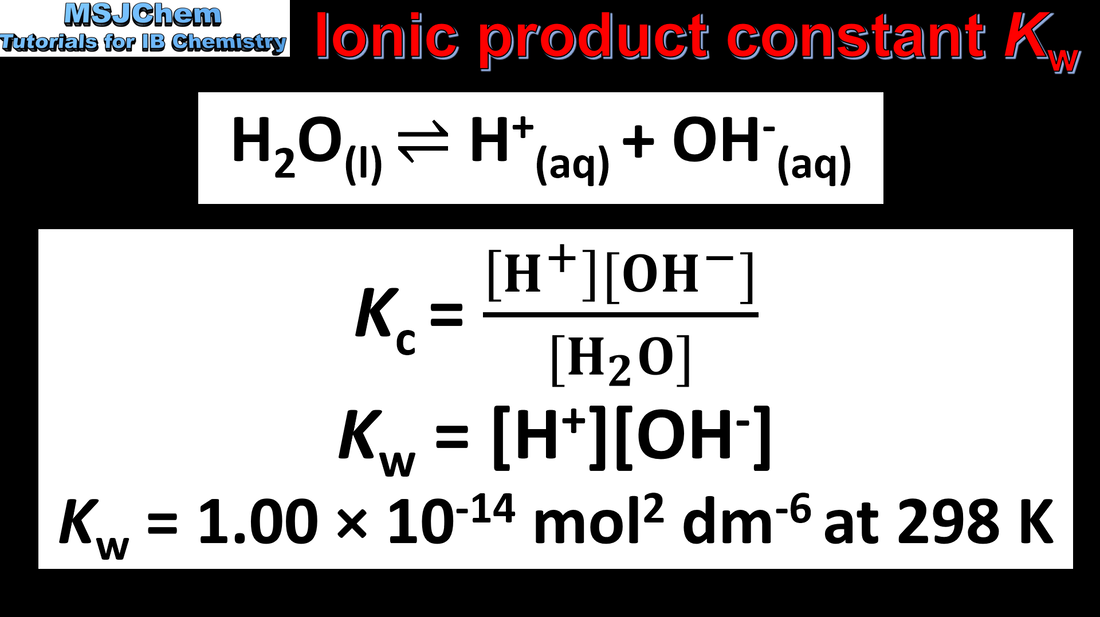
8.3 Ionic product constant of water Kw
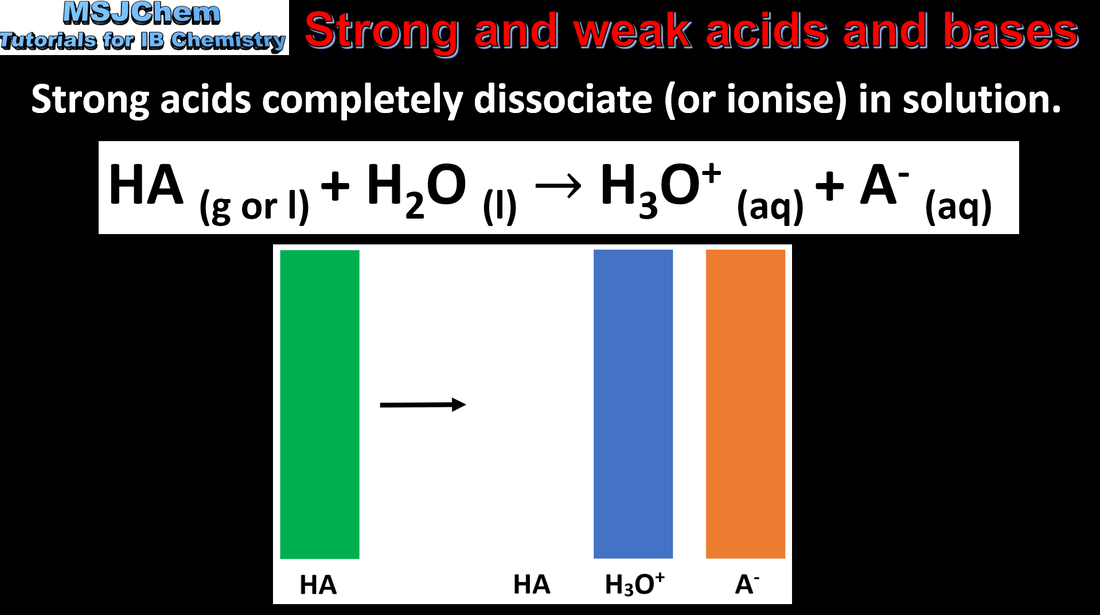
8.4 Strong and weak acids and bases
8.4 Strong and weak acids and bases
8.5 Acid deposition
|
Understandings:
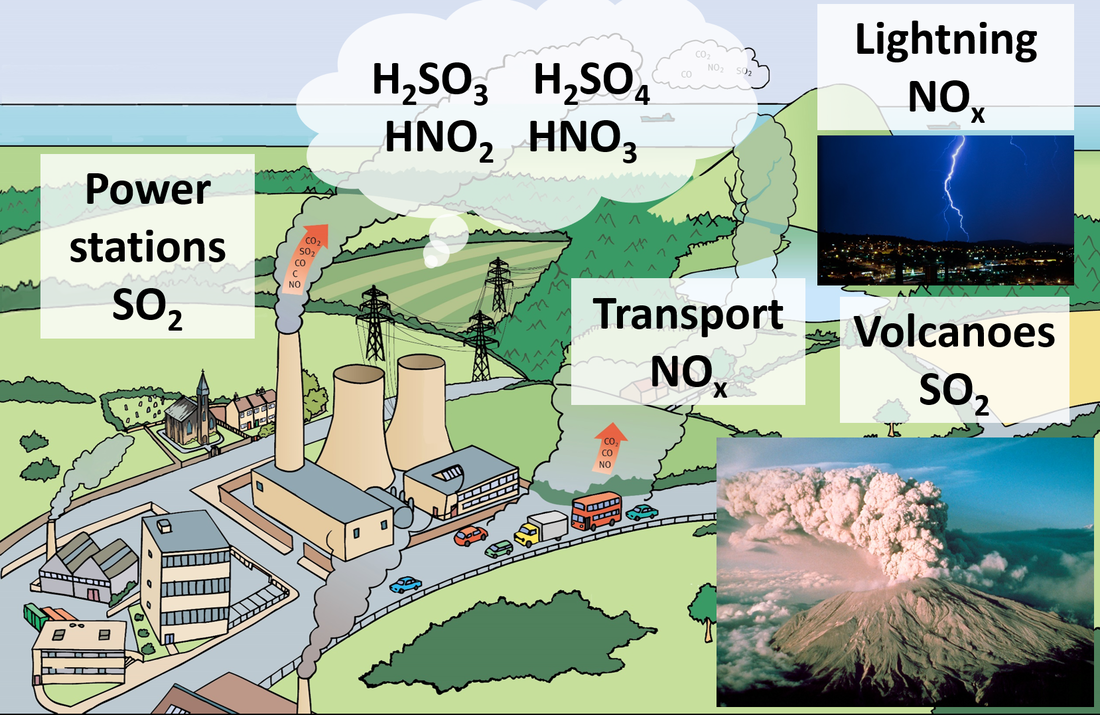
Rain is naturally acidic because of dissolved CO2 and has a pH of 5.6. Acid deposition has a pH below 5.0 Acid deposition is formed when nitrogen or sulfur oxides dissolve in water to form HNO3, HNO2, H2SO4 and H2SO3. Sources of the oxides of sulfur and nitrogen should be covered. Guidance: Balancing the equations that describe the combustion of sulfur and nitrogen to their oxides and the subsequent formation of H2SO3, H2SO4, HNO2 and HNO3. |
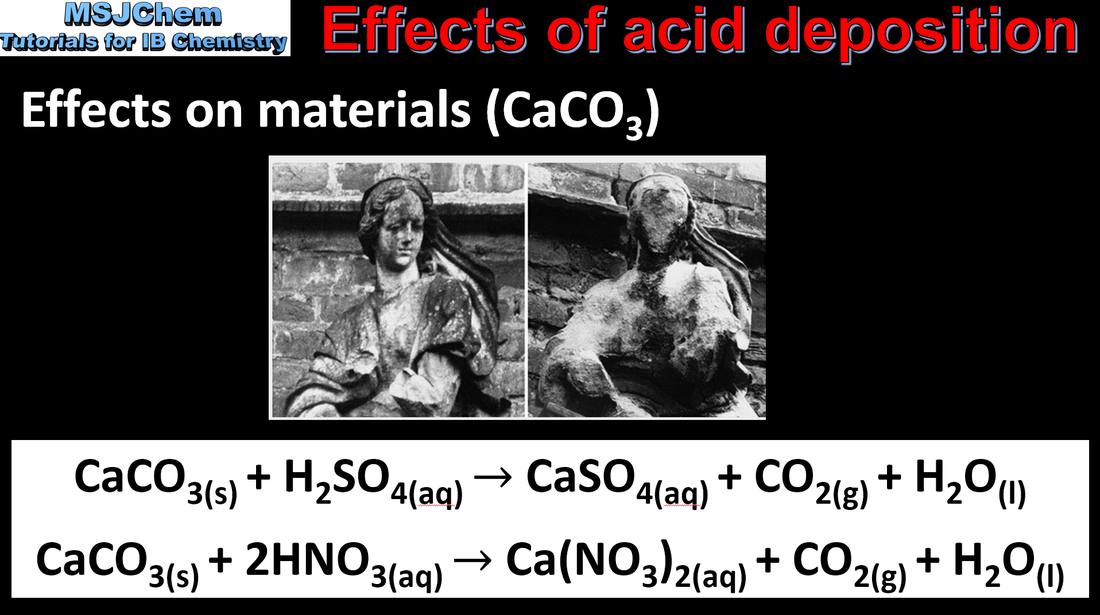
8.5 Effects and reduction of acid deposition
|
Understandings:
The effects of acid deposition should be covered. Applications and skills: Distinction between the pre-combustion and post-combustion methods of reducing sulfur oxides emissions. Deduction of acid deposition equations for acid deposition with reactive metals and carbonates. https://youtu.be/WUULrLtBhfg |
Further details about hydrodesulfurization: The oil based raw material is heated to 300-400°C and pumped under a pressure of up to 130 atm into a hydrodesulfurization reactor. The mixture passes over a catalyst which breaks the sulfur-carbon bonds, allowing the sulfur to react with the hydrogen to form hydrogen sulfide (H2S)