Structure 3.1 The periodic table: Classification of elements (HL)
Structure 3.1.7
Understandings:
Understandings:
- Discontinuities occur in the trend of increasing first ionisation energy across a period.
- Explain how these discontinuities provide evidence for the existence of energy sublevels.
- Explanations should be based on the energy of the electron removed, rather than on the “special stability” of filled and half-filled sublevels.
Structure 3.1.8
Understandings:
Understandings:
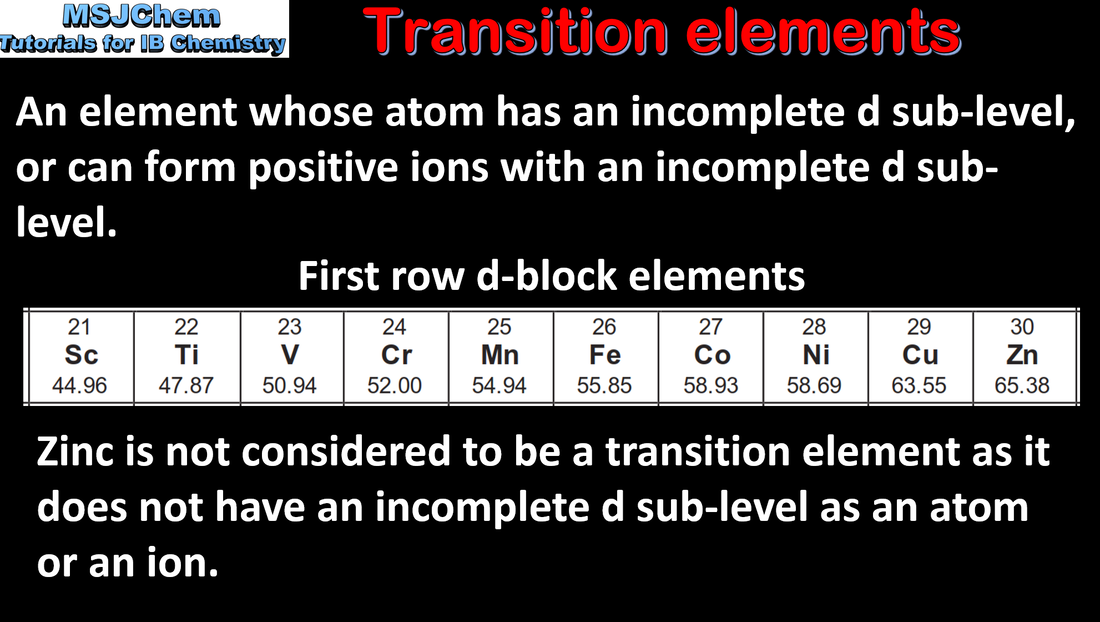
- Transition elements have incomplete d-sublevels that give them characteristic properties.
- Recognize properties, including: variable oxidation state, high melting points, magnetic properties, catalytic properties, formation of coloured compounds and formation of complex ions with ligands.
- Knowledge of different types of magnetism will not be assessed.
- Structure 2.3 What are the arguments for and against including scandium as a transition element?
Structure 3.1.9
Understandings:
Understandings:
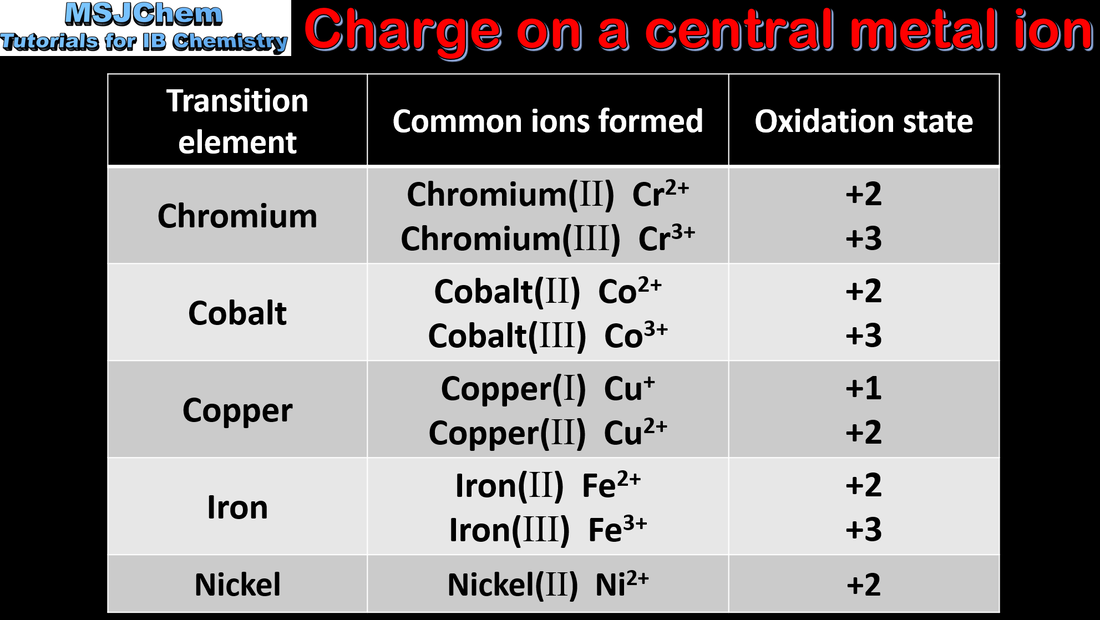
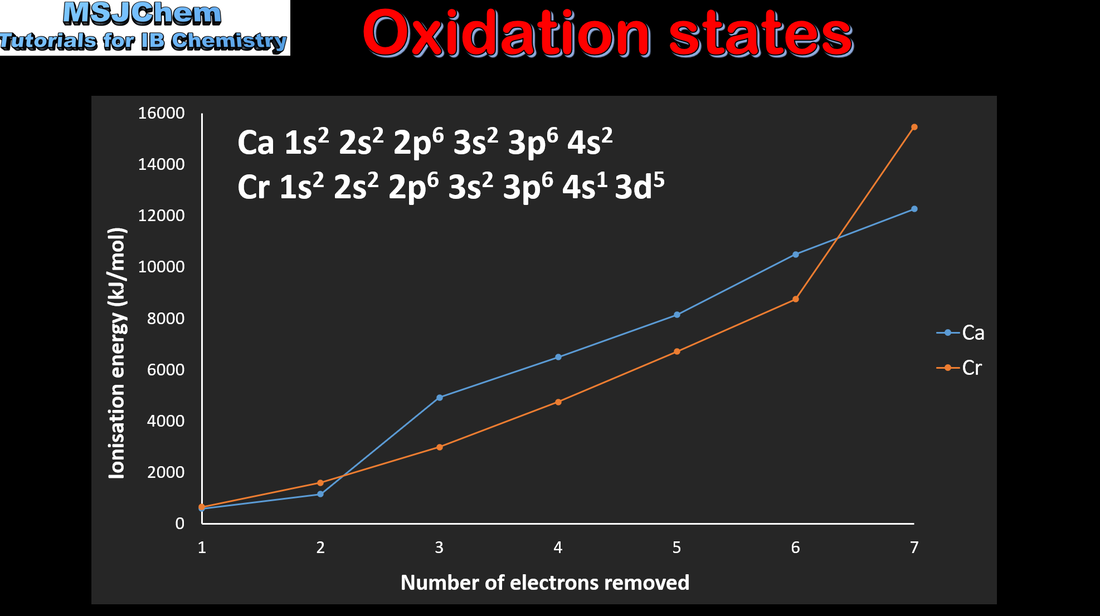
- The formation of variable oxidation states in transition elements can be explained by the fact that their successive ionisation energies are close in value.
- Deduce the electron configurations of ions of the first-row transition elements.
Structure 3.1.10
Understandings:
Understandings:
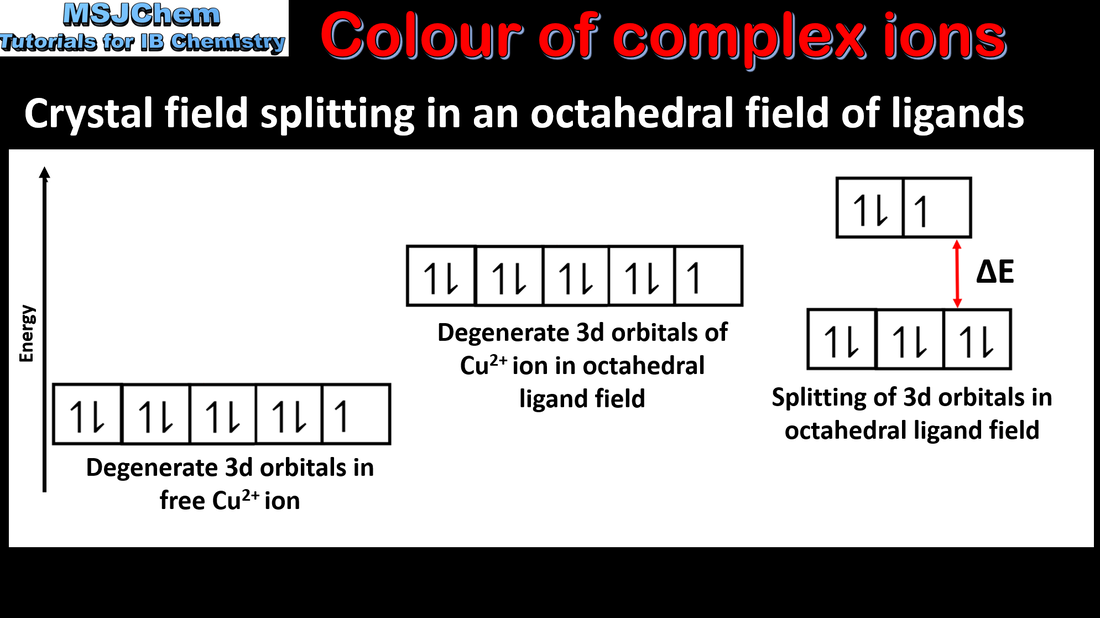
- Transition element complexes are coloured due to the absorption of light when an electron is promoted between the orbitals in the split d-sublevels. The colour absorbed is complementary to the colour observed.
- Apply the colour wheel to deduce the wavelengths and frequencies of light absorbed and/or observed.
- The colour wheel and the equation c = λf are given in the data booklet.
- Students are not expected to know the different splitting patterns and their relation to the coordination number.
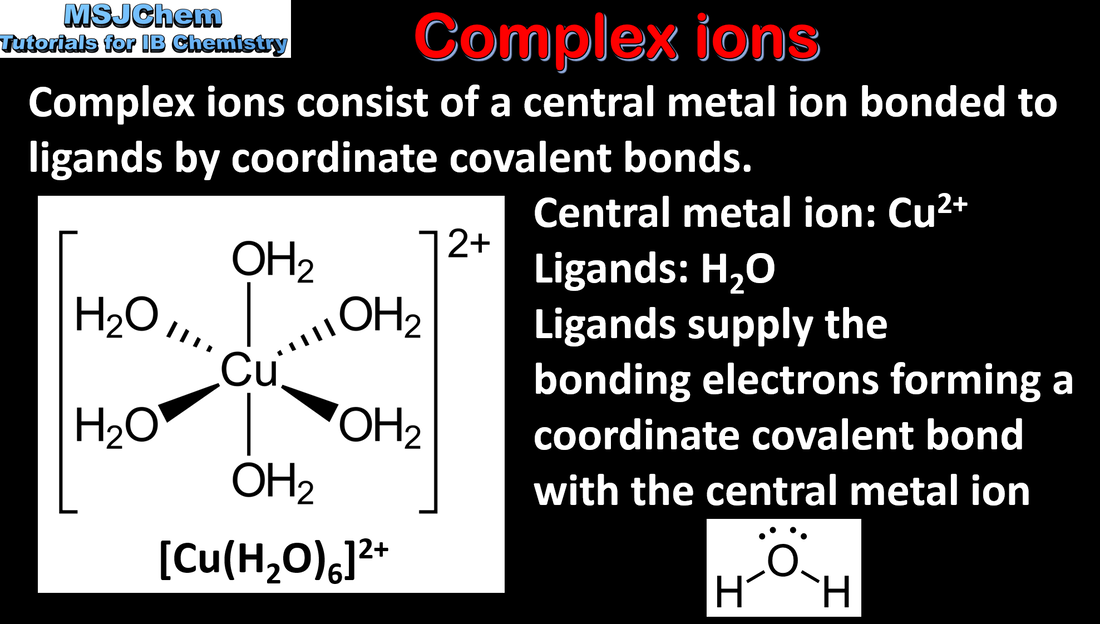
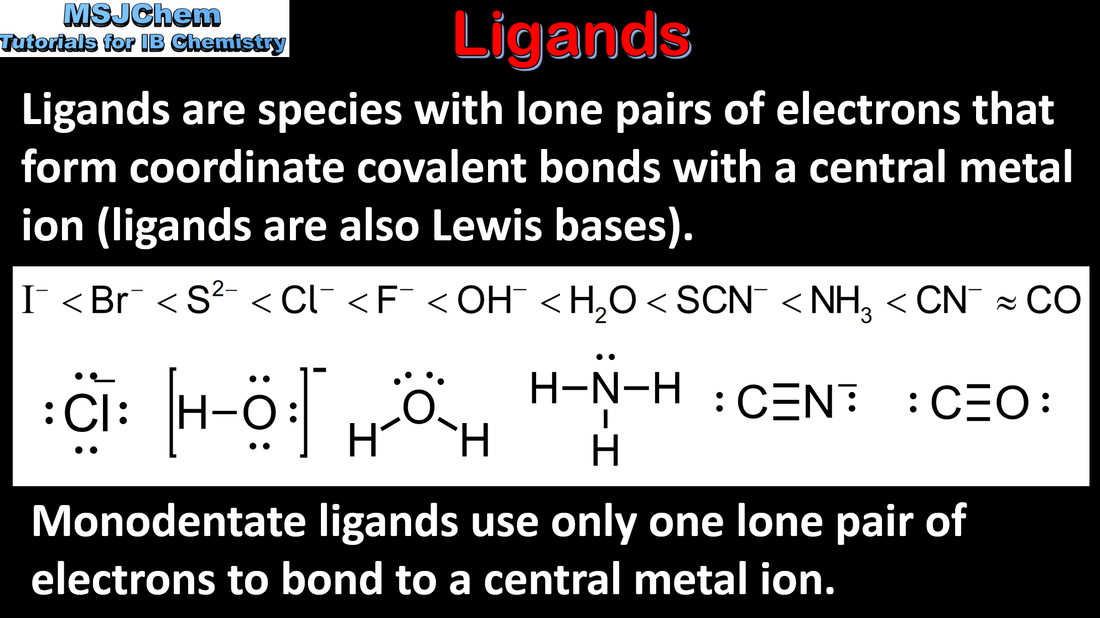
- Reactivity 3.4 What is the nature of the reaction between transition element ions and ligands in forming complex ions?