Structure 3.2 Functional groups: Classification of organic compounds
Structure 3.2.1
Understandings:
Understandings:
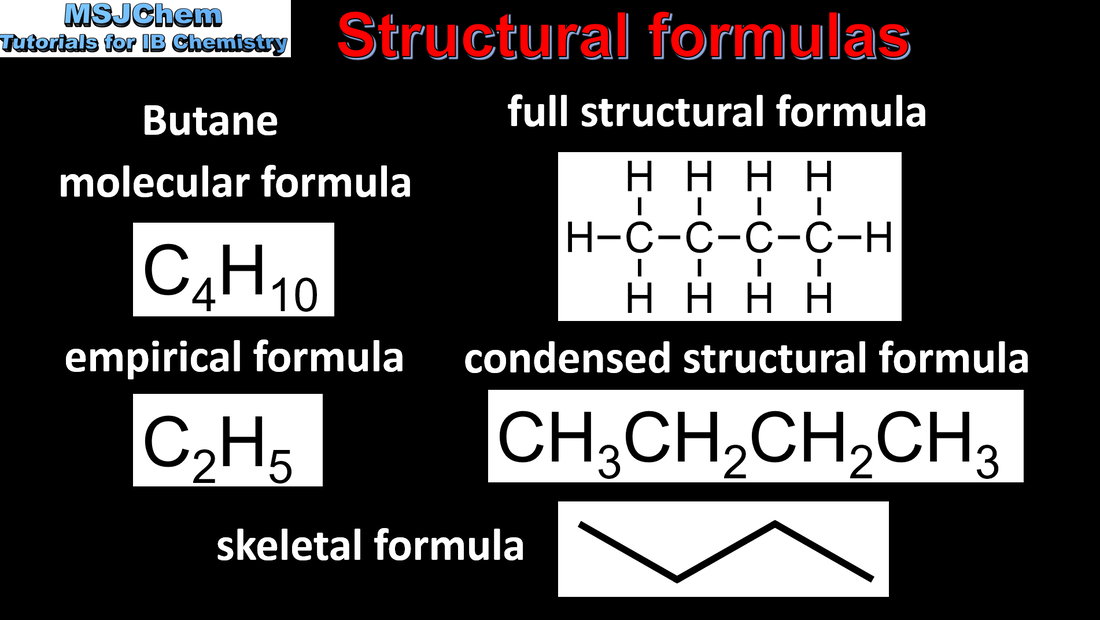
- Organic compounds can be represented by different types of formulas. These include empirical, molecular, structural (full and condensed), stereochemical and skeletal.
- Identify different formulas and interconvert molecular, skeletal and structural formulas.
- Construct 3D models (real or virtual) of organic molecules.
- Stereochemical formulas are not expected to be drawn, except where specifically indicated.
- Structure 2.2 What is unique about carbon that enables it to form more compounds than the sum of all the other elements’ compounds?
Structure 3.2.2
Understandings:
Understandings:
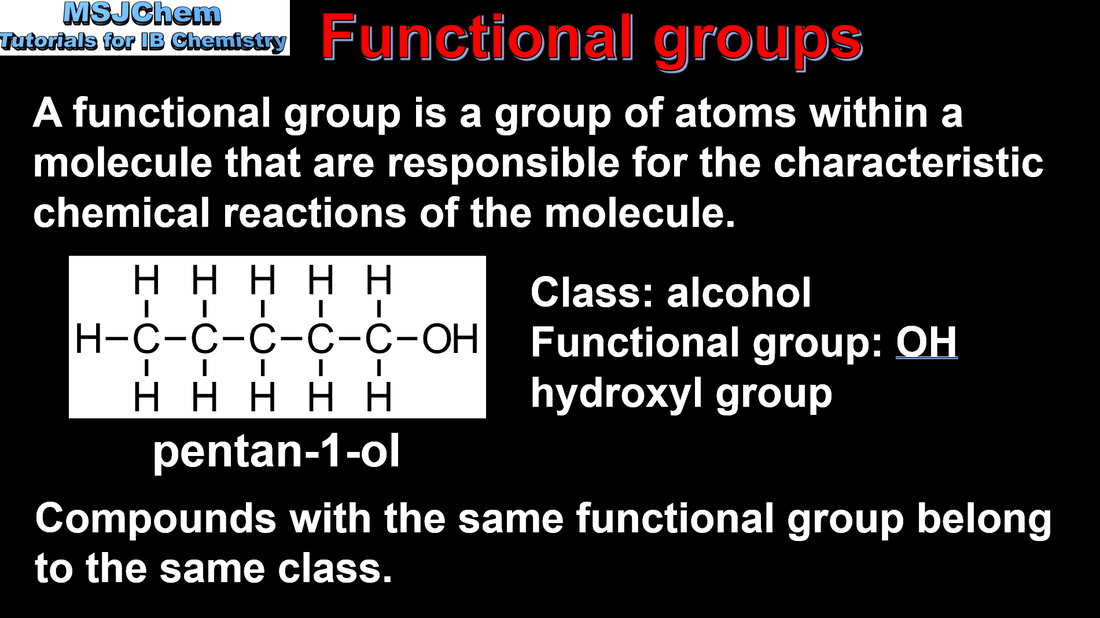
- Functional groups give characteristic physical and chemical properties to a compound. Organic compounds are divided into classes according to the functional groups present in their molecules.
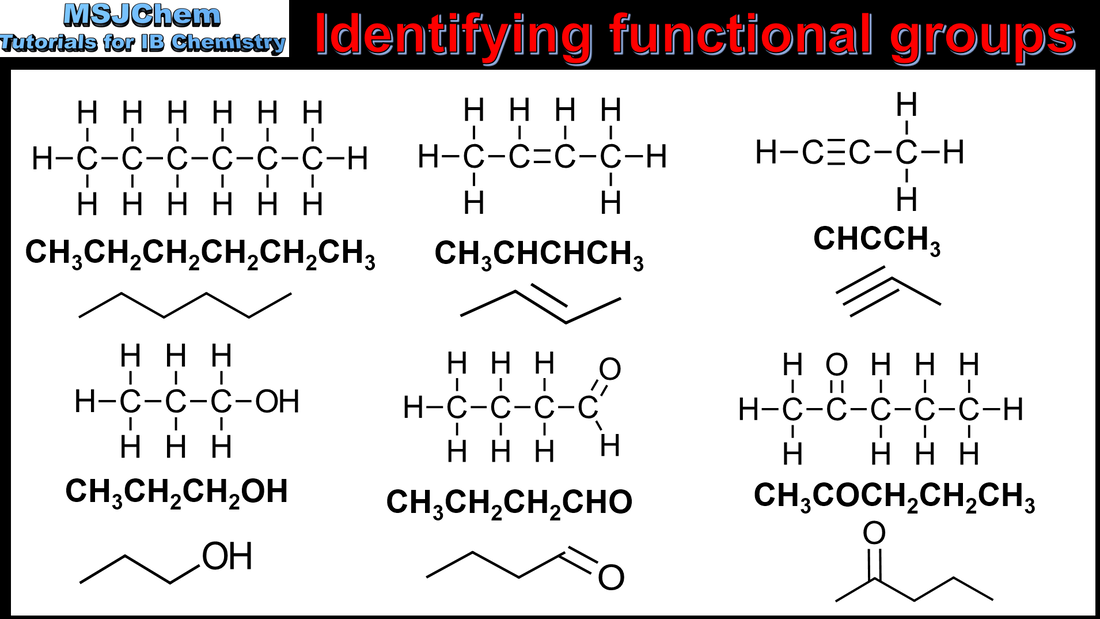
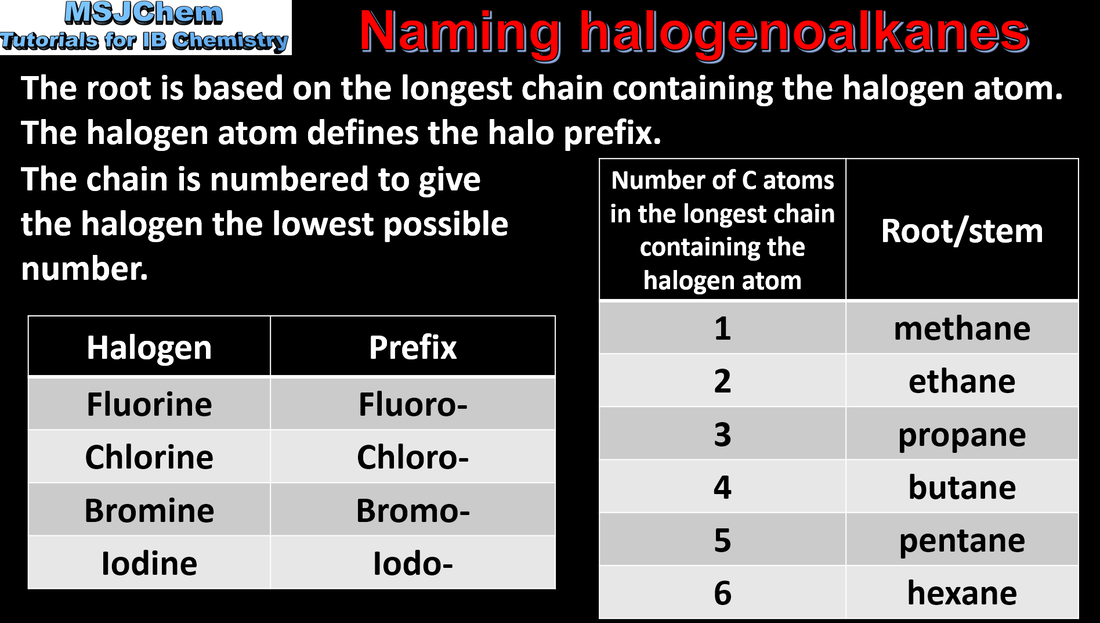
- Identify the following functional groups by name and structure: halogeno, hydroxyl, carbonyl, carboxyl, alkoxy, amino, amido, ester, phenyl.
- The terms “saturated” and “unsaturated” should be included.
- Structure 2.4 (HL) What is the nature of the reaction that occurs when two amino acids form a dipeptide?
Structure 3.2.3 and 3.2.4
Understandings:
Understandings:
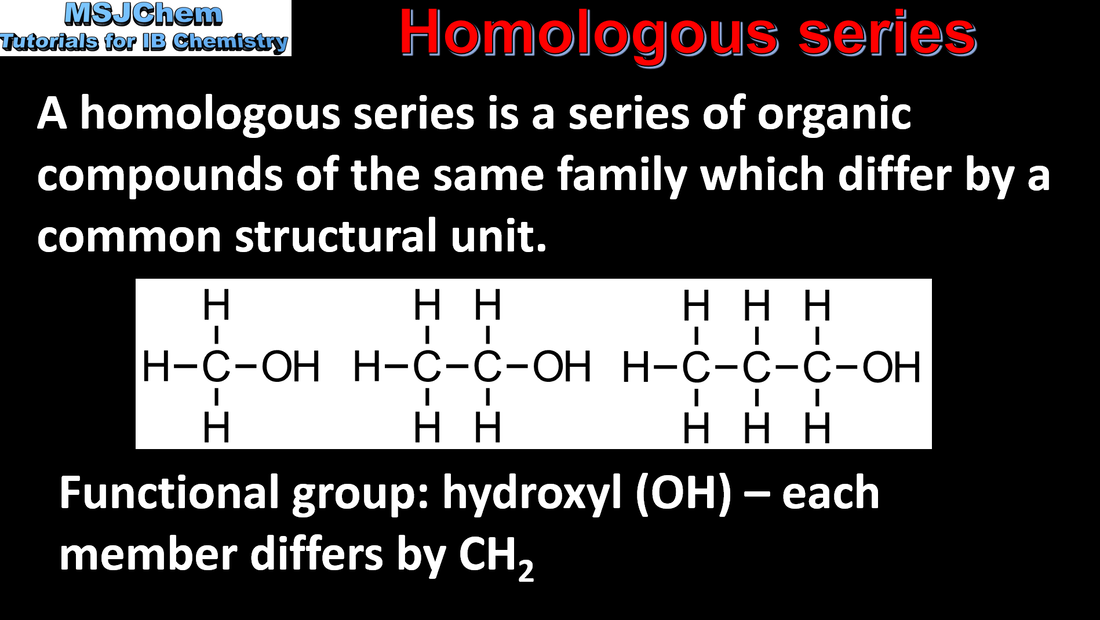
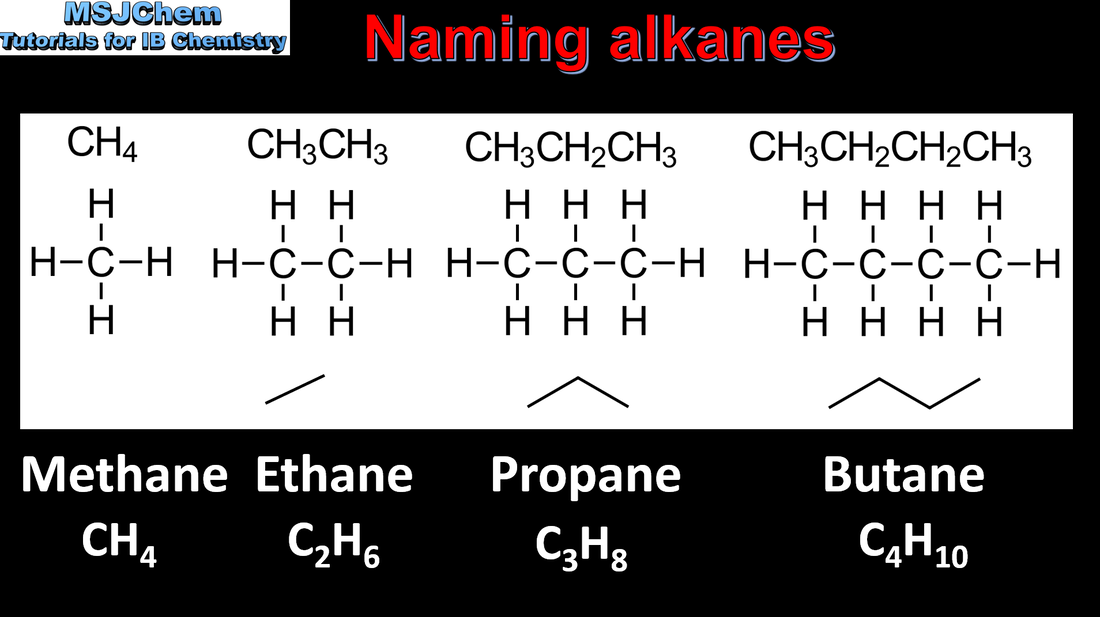
- A homologous series is a family of compounds in which successive members differ by a common structural unit, typically CH2. Each homologous series can be described by a general formula (3.2.3).
- Successive members of a homologous series show a trend in physical properties (3.2.4).
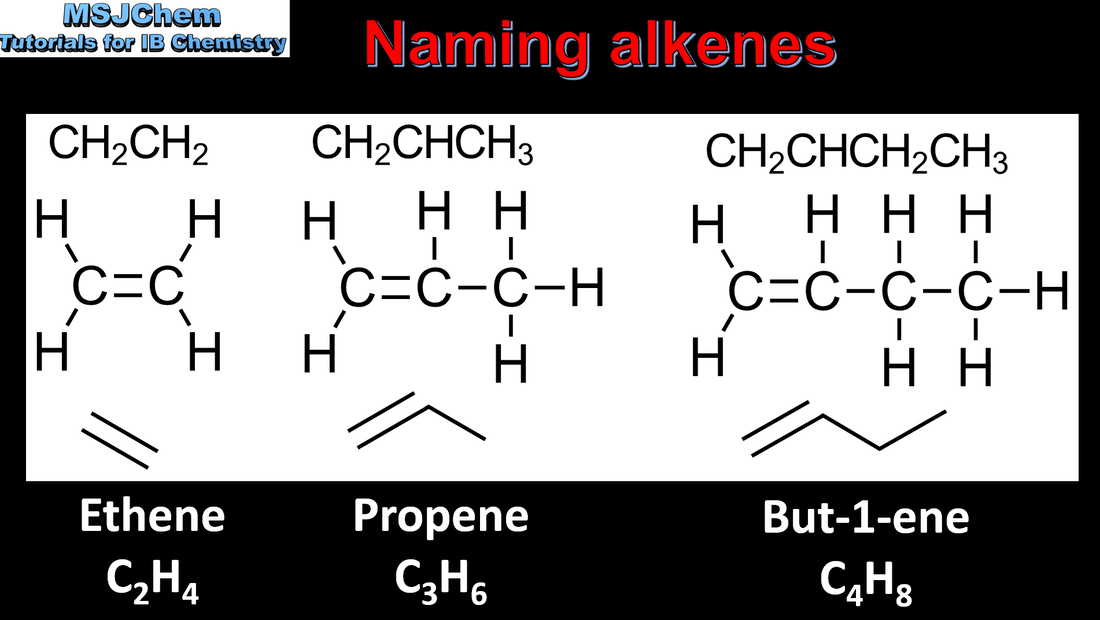
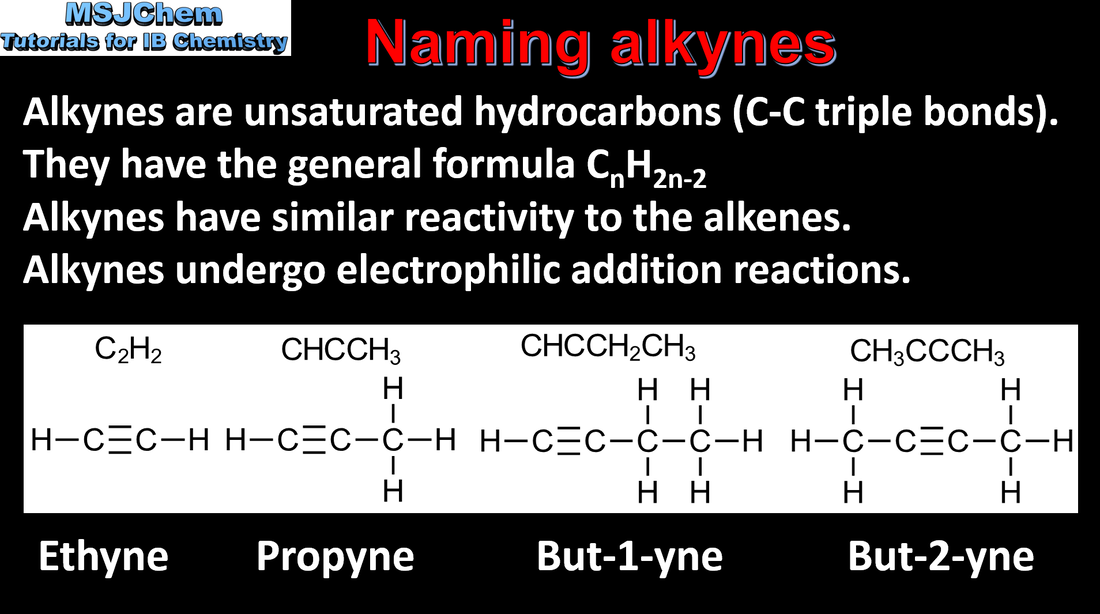
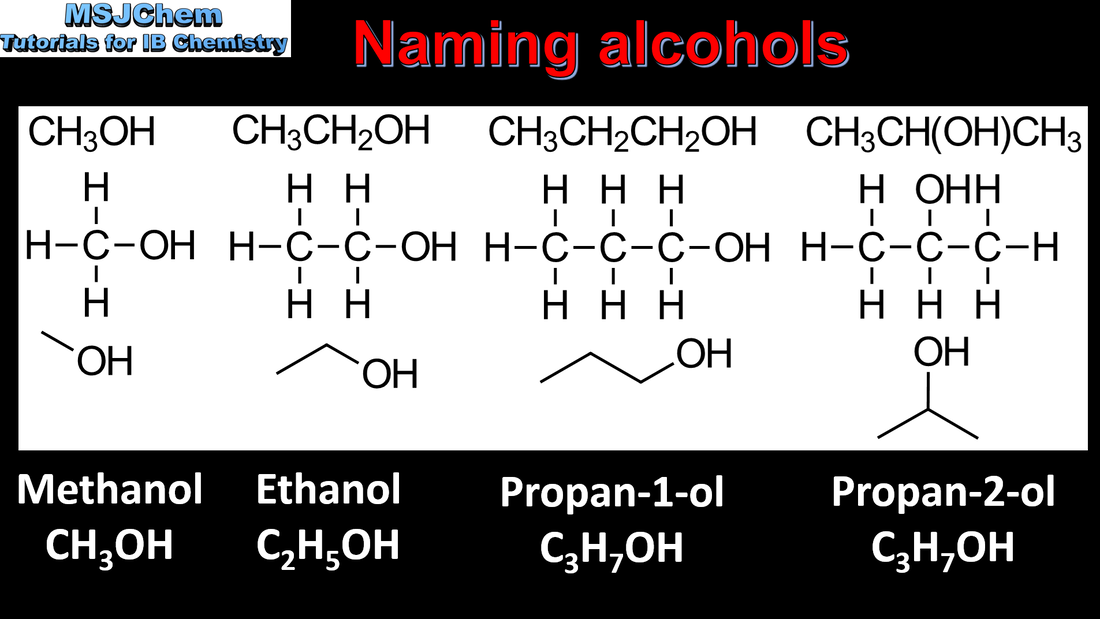
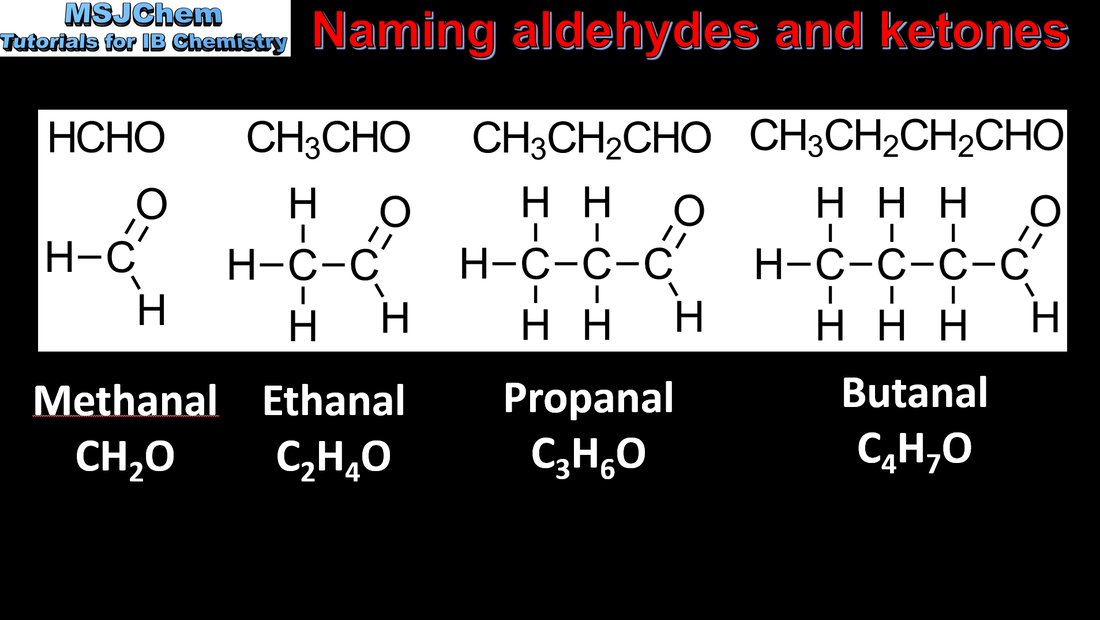
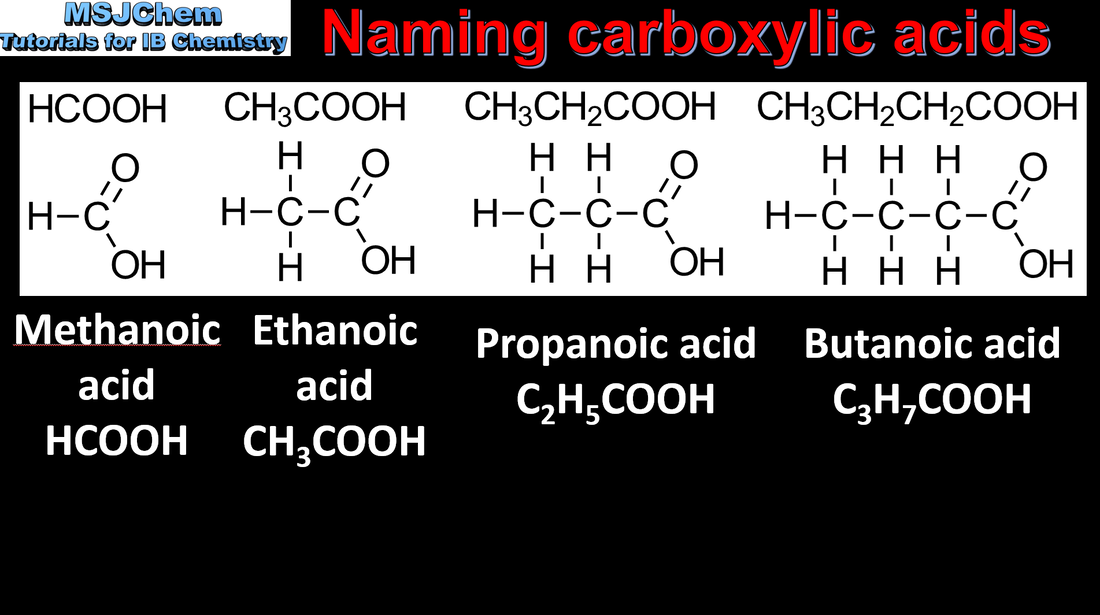
- Identify the following homologous series: alkanes, alkenes, alkynes, halogenoalkanes, alcohols, aldehydes, ketones, carboxylic acids, ethers, amines, amides and esters (3.2.3).
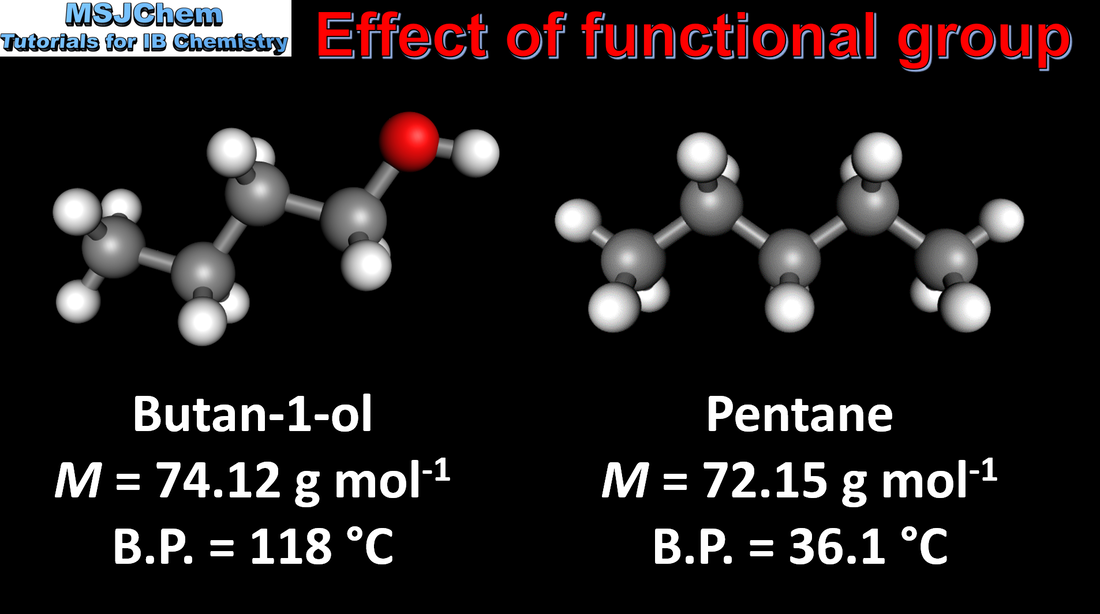
- Describe and explain the trend in melting and boiling points of members of a homologous series (3.2.4).
- The terms “saturated” and “unsaturated” should be included.
- Structure 2.4 (HL) What is the nature of the reaction that occurs when two amino acids form a dipeptide?
- Structure 2.2 What is the influence of the carbon chain length, branching and the nature of the functional groups on intermolecular forces?
Structure 3.2.5
Understandings:
Understandings:
- IUPAC nomenclature refers to a set of rules used by the International Union of Pure and Applied Chemistry to apply systematic names to organic and inorganic compounds.
- Apply IUPAC nomenclature to saturated or mono-unsaturated compounds that have up to six carbon atoms in the parent chain and contain one type of the following functional groups: halogeno, hydroxyl, carbonyl, carboxyl.
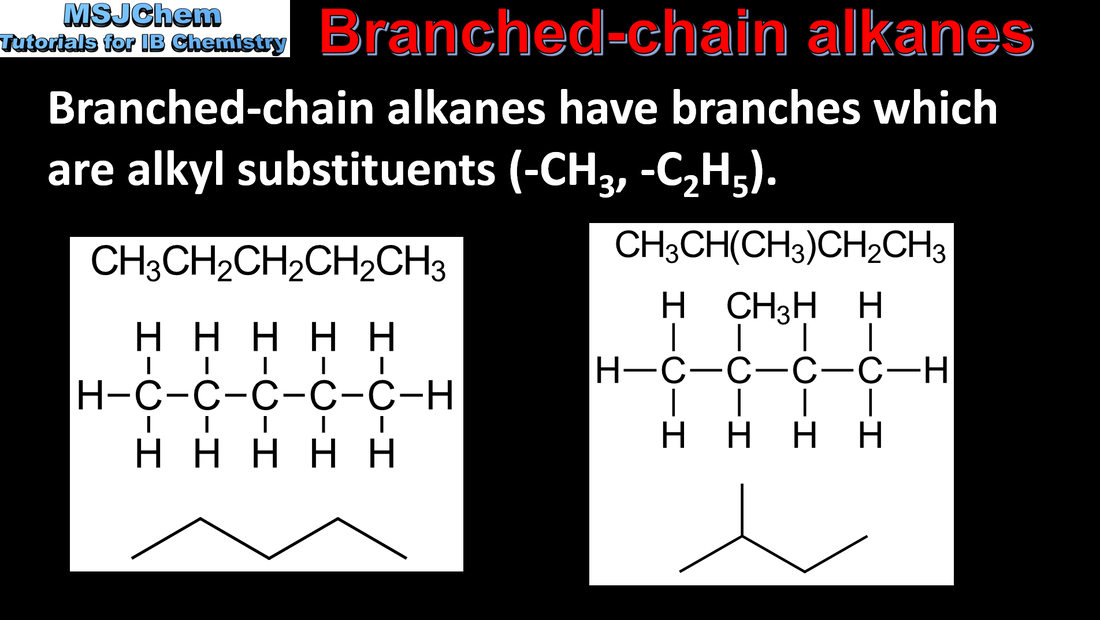
- Include straight-chain and branched-chain isomers.
Structure 3.2.6
Understandings:
Understandings:
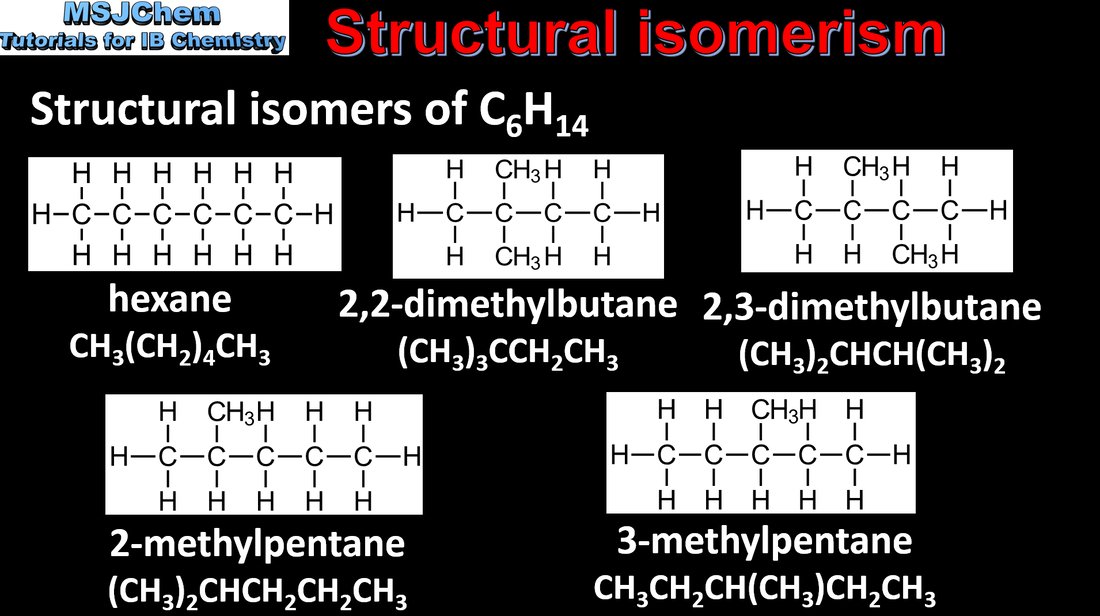
- Structural isomers are molecules that have the same molecular formula but different connectivities.
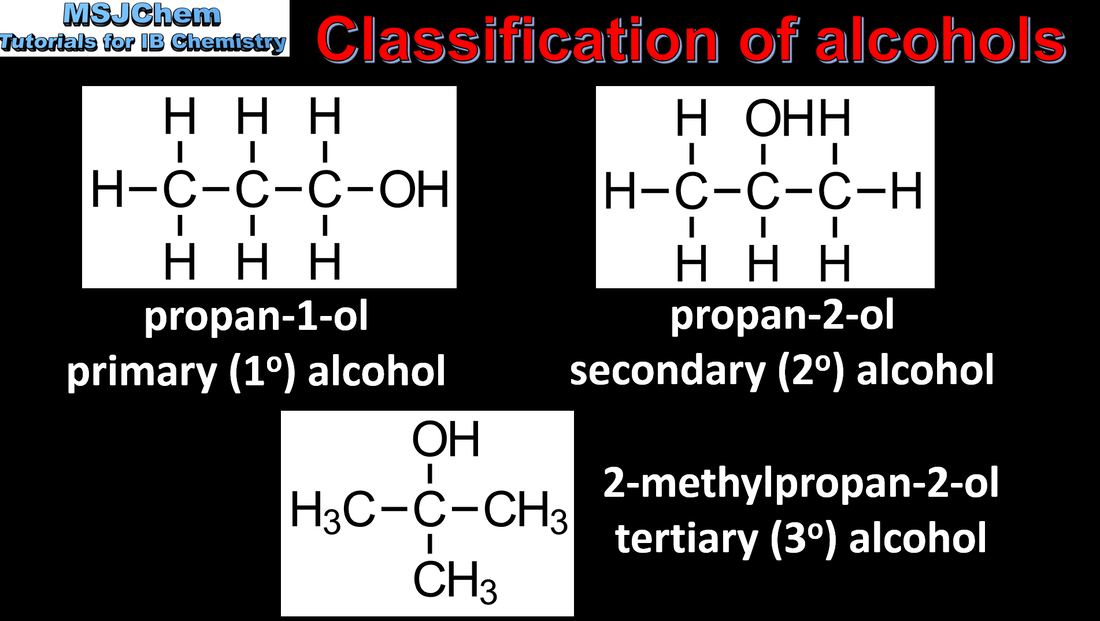
- Recognise isomers, including branched, straight-chain, position and functional group isomers.
- Primary, secondary and tertiary alcohols, halogenoalkanes and amines should be included.
- Structure 2.2 (HL) How does the fact that there are only 3 isomers of dibromobenzene support the current model of benzene’s structure?