Structure 1.3 Electron configurations
Structure 1.3.1
Understandings:
Understandings:
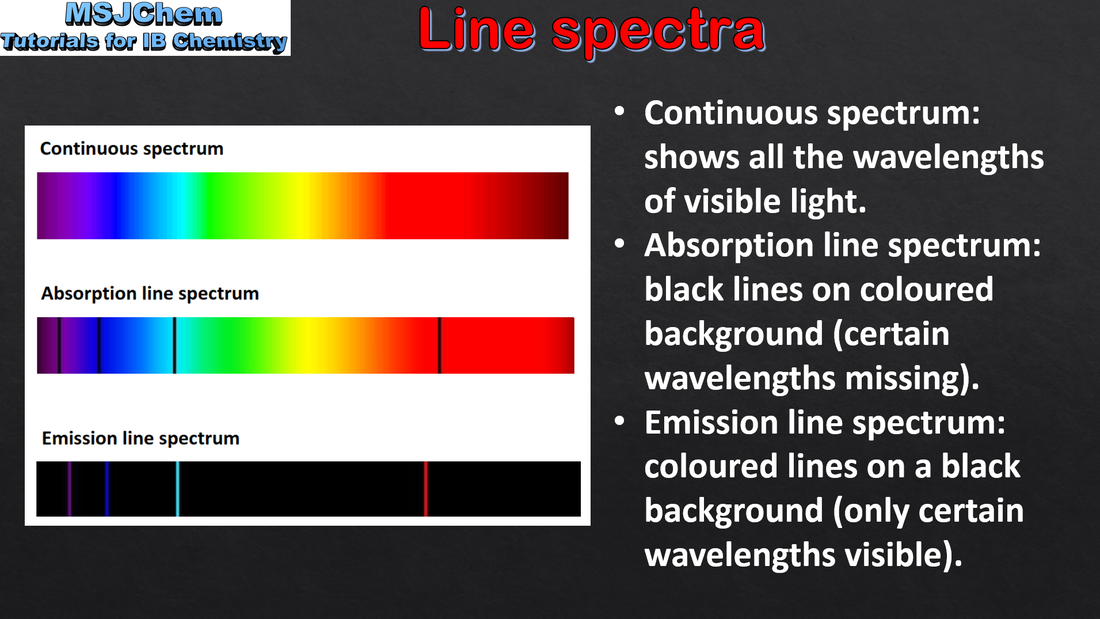
- Emission spectra are produced by atoms emitting photons when electrons in excited states return to lower energy levels.
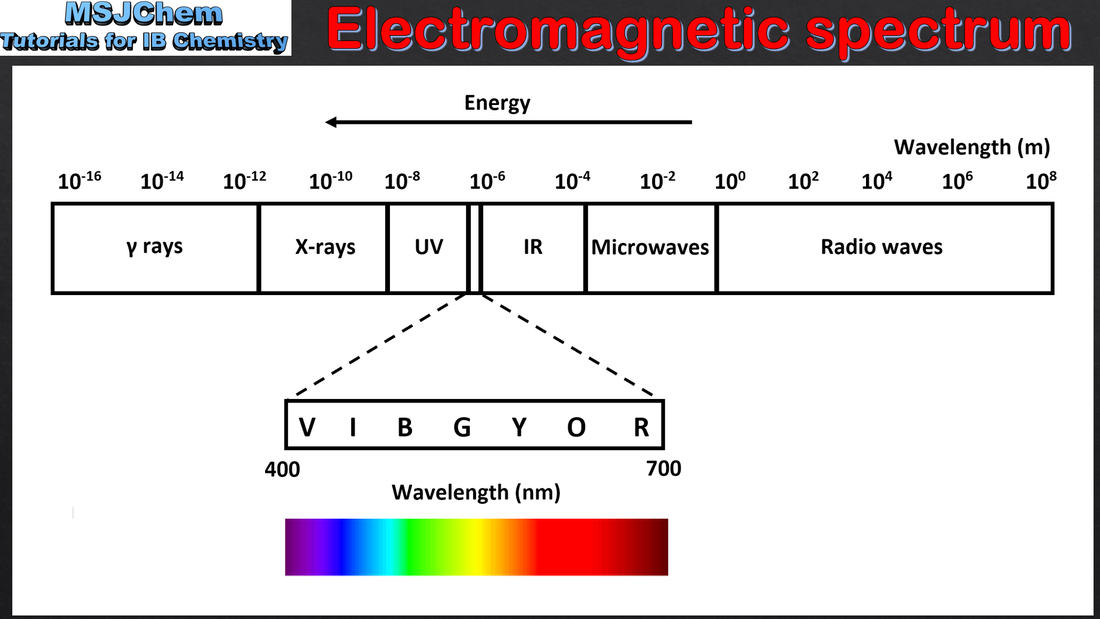
- Qualitatively describe the relationship between colour, wavelength, frequency and energy across the electromagnetic spectrum.
- Distinguish between a continuous and a line spectrum.
Structure 1.3.2
Understandings:
Understandings:
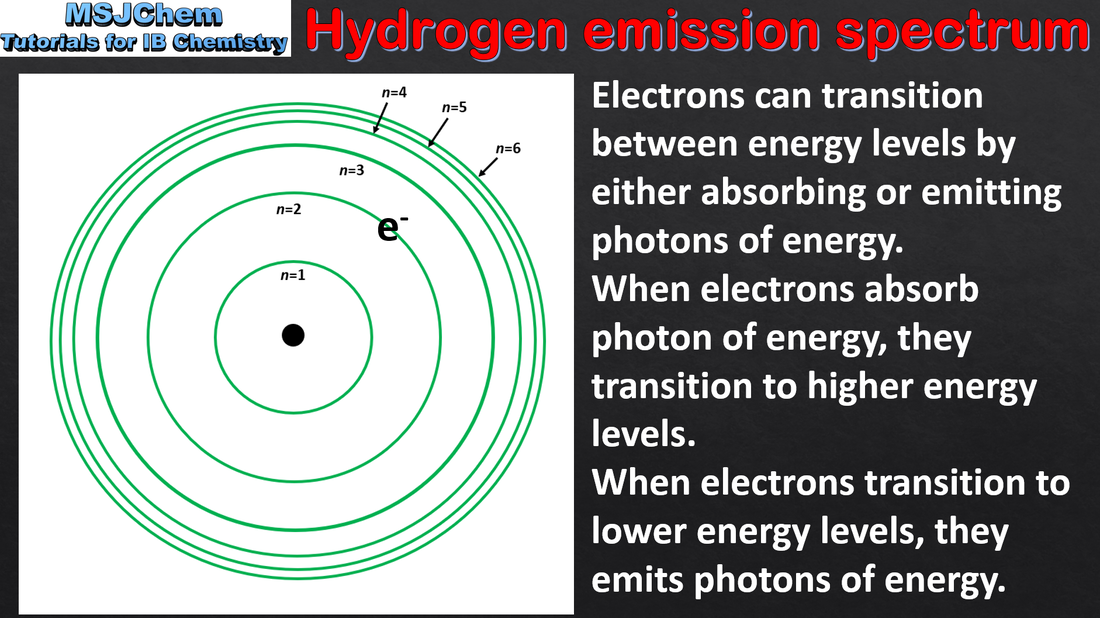
- The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies.
- Describe the emission spectrum of the hydrogen atom, including the relationships between the lines and energy transitions to the first, second and third energy levels.
Structure 1.3.3 and 1.3.4
Understandings:
Understandings:
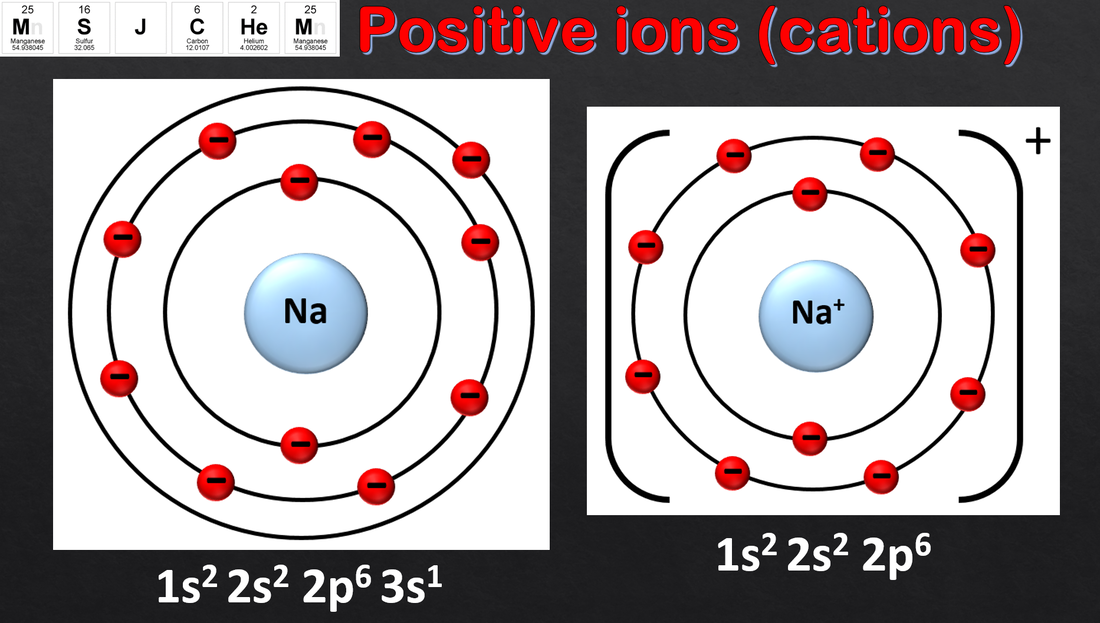
- The main energy level is given an integer number, n, and can hold a maximum of 2n2 electrons (1.3.3).
- A more detailed model of the atom describes the division of the main energy level into s, p, d and f sublevels of successively higher energies (1.3.4).
- Deduce the maximum number of electrons that can occupy each energy level (1.3.3).
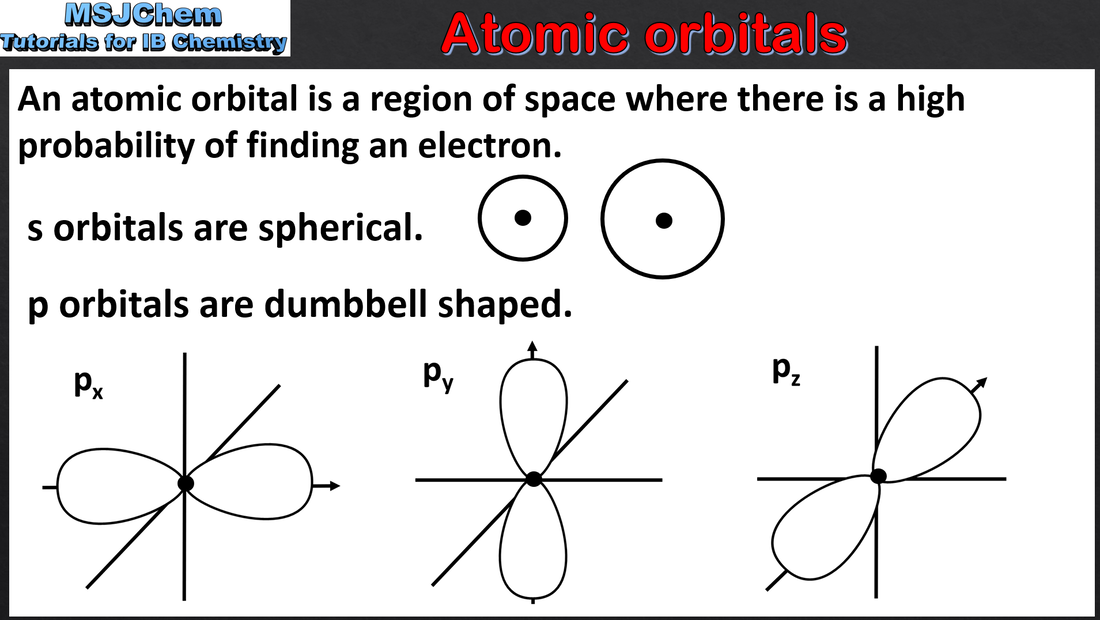
- Recognize the shape and orientation of an s atomic orbital and the three p atomic orbitals (1.3.4).
Structure 1.3.5
Understandings:
Understandings:
- Each orbital has a defined energy state for a given electron configuration and chemical environment, and can hold two electrons of opposite spin.
- Sublevels contain a fixed number of orbitals, regions of space where there is a high probability of finding an electron.
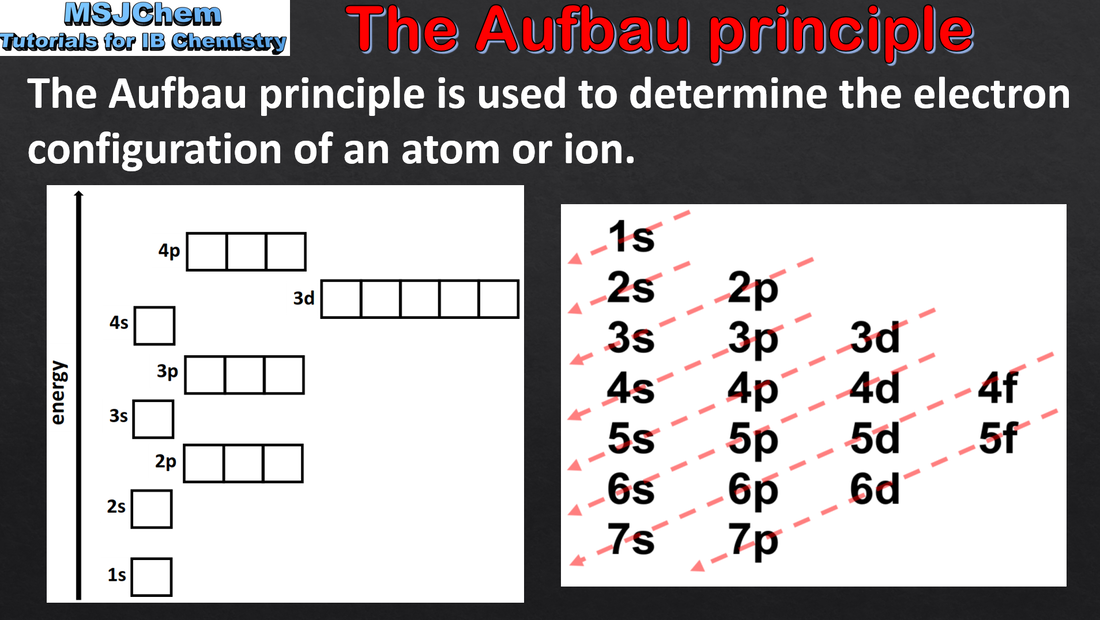
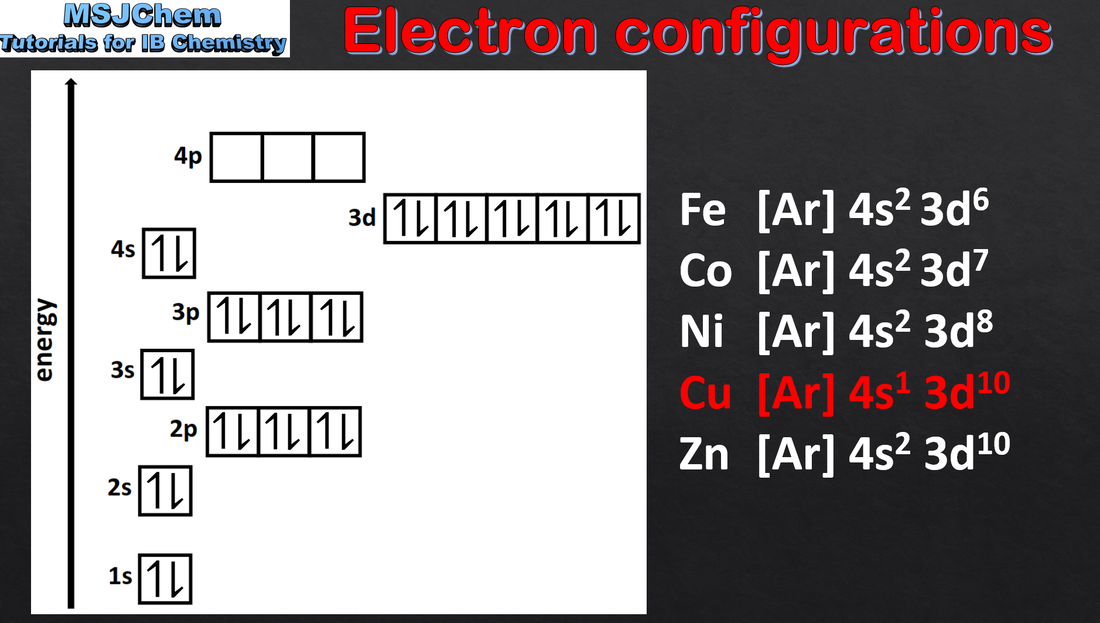
- Apply the Aufbau principle, Hund’s rule and the Pauli exclusion principle to deduce electron configurations for atoms and ions up to Z = 36.
- Full electron configurations and condensed electron configurations using the noble gas core should be covered.
- Orbital diagrams, i.e. arrow-in-box diagrams, should be used to represent the filling and relative energy of orbitals.
- The electron configurations of Cr and Cu as exceptions should be covered.
The following two videos cover how to write electron configurations for atoms with atomic numbers 1 to 36.