Structure 2.2 The covalent model (HL)
Structure 2.2.11
Understandings:
Understandings:
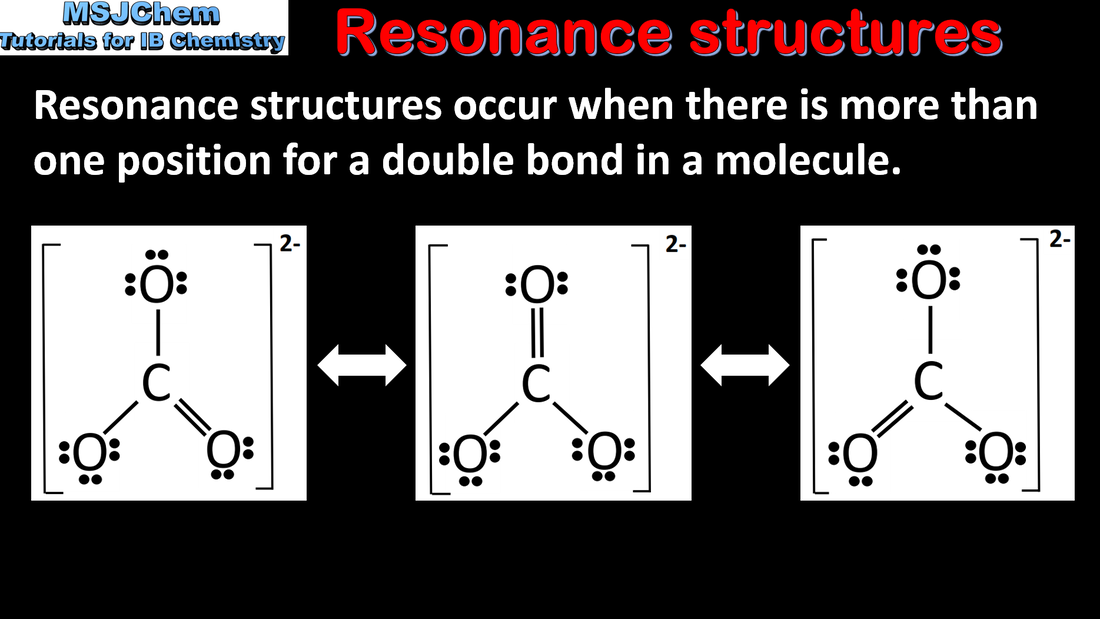
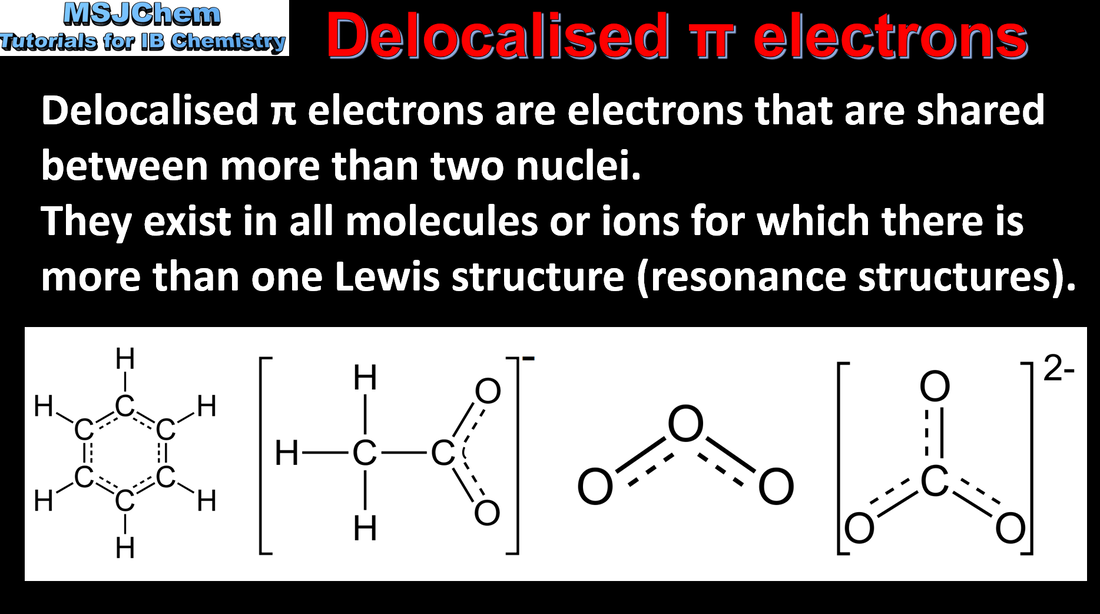
- Resonance structures occur when there is more than one possible position for a double bond in a molecule.
- Deduce resonance structures of molecules and ions.
- Include the term “delocalisation”.
- Structure 1.3 Why are oxygen and ozone dissociated by different wavelengths of light?
Structure 2.2.12
Understandings:
Understandings:
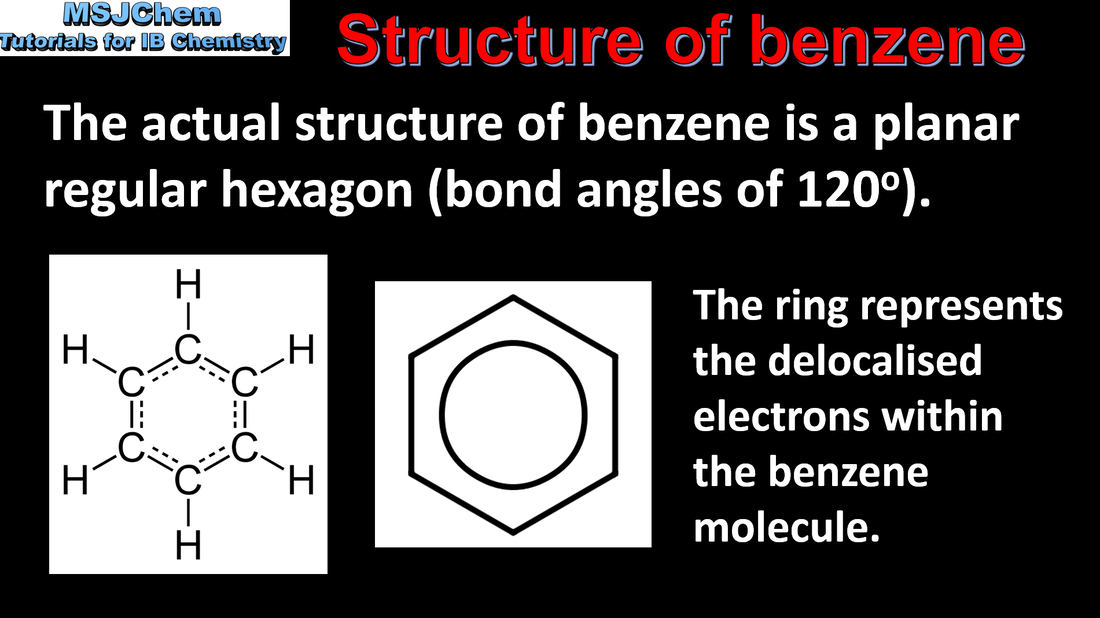
- Benzene, C6H6, is an important example of a molecule that has resonance.
- Discuss the structure of benzene from physical and chemical evidence.
- Reactivity 2.1, 2.2 How does the resonance energy in benzene explain its relative unreactivity?
- Reactivity 3.4 What are the structural features of benzene that favour it undergoing electrophilic substitution reactions?
Structure 2.2.13
Understandings:
Understandings:
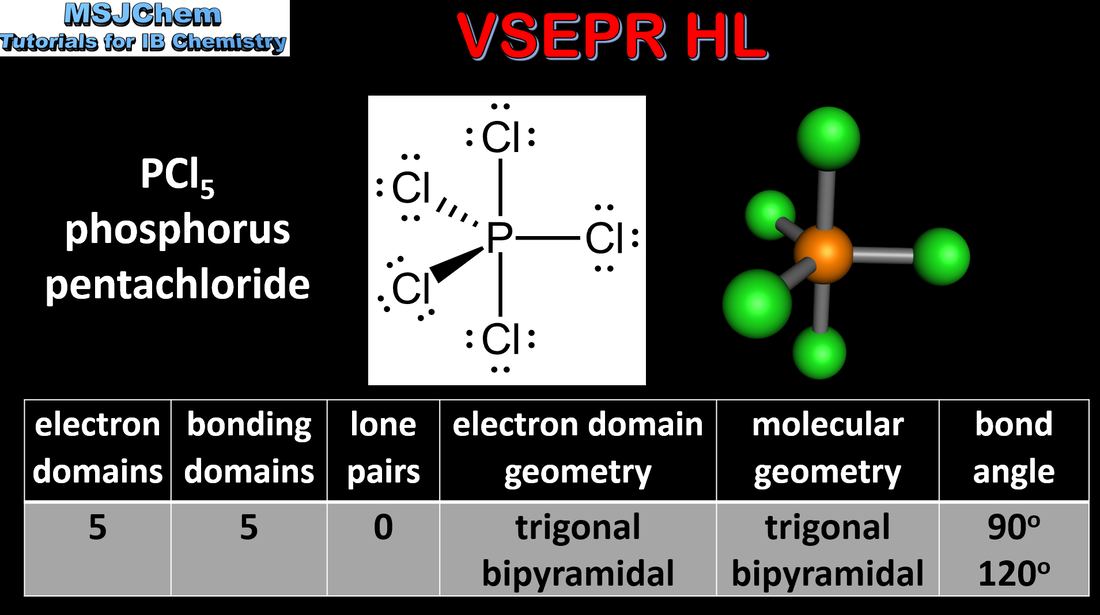
- Some atoms can form molecules in which they have an expanded octet of electrons.
- Represent Lewis formulas for species with five and six electron domains around the central atom.
- Deduce the electron domain geometry and the molecular geometry for these species using the VSEPR model.
- Structure 3.1 How does the ability of some atoms to expand their octet relate to their position in the periodic table?
Structure 2.2.14
Understandings:
Understandings:
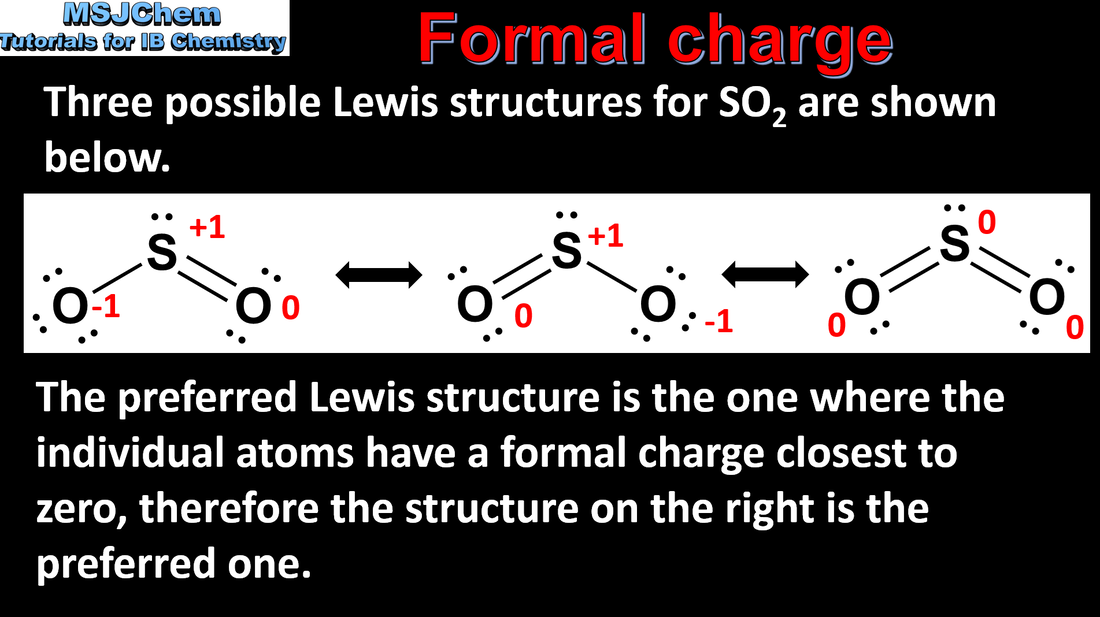
- Formal charge values can be calculated for each atom in a species and used to determine which of several possible Lewis formulas is preferred.
- Apply formal charge to determine a preferred Lewis formula from different Lewis formulas for a species.
- Structure 3.1, Reactivity 3.2 What are the different assumptions made in the calculation of formal charge and of oxidation states for atoms in a species?
Structure 2.2.15
Understandings:
Understandings:
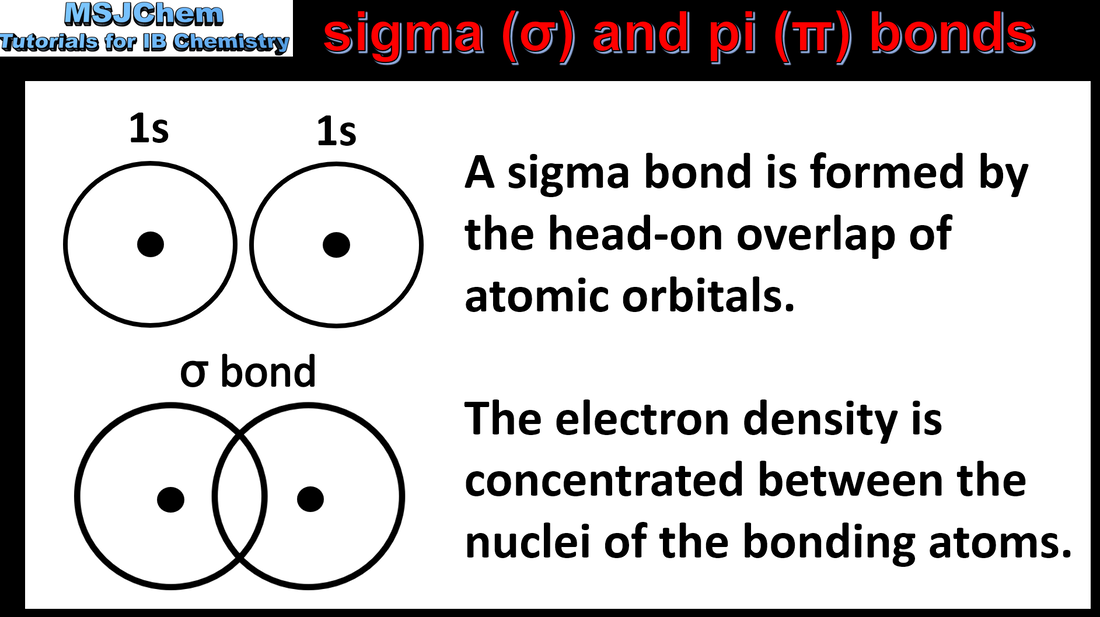
- Sigma bonds σ form by the head-on combination of atomic orbitals where the electron density is concentrated along the bond axis.
- Pi bonds π form by the lateral combination of p-orbitals where the electron density is concentrated on opposite sides of the bond axis.
- Deduce the presence of sigma bonds and pi bonds in molecules and ions.
- Include both organic and inorganic examples.
Structure 2.2.16
Understandings:
Understandings:
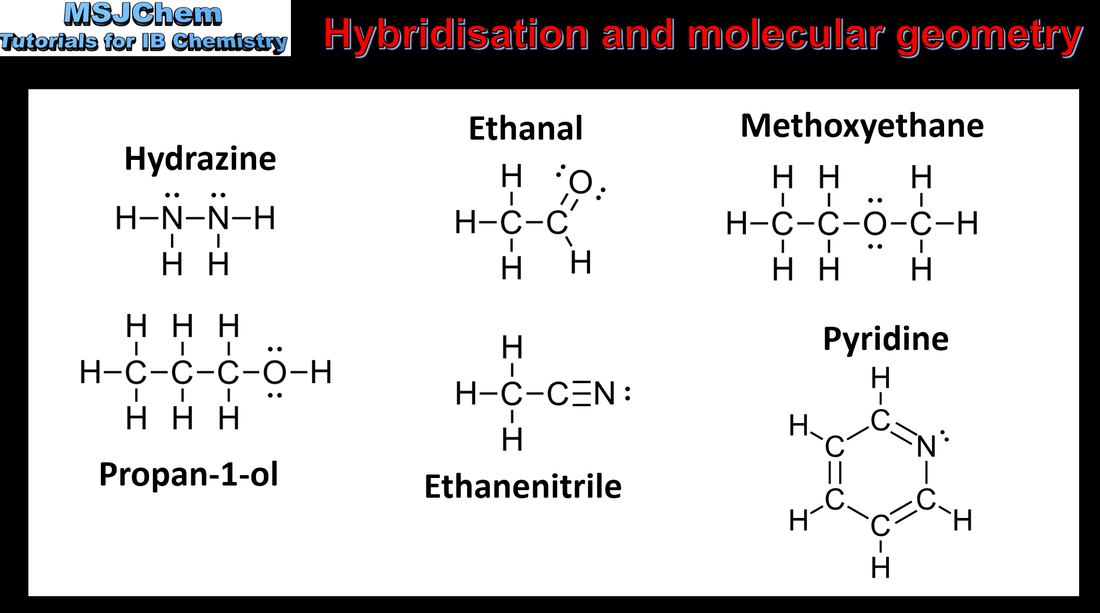
- Hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals for bonding.
- Analyse the hybridization and bond formation in molecules and ions.
- Identify the relationships between Lewis formulas, electron domains, molecular geometry and type of hybridization.
- Predict the geometry around an atom from its hybridization, and vice versa.
- Include both organic and inorganic examples. Only sp, sp2 and sp3 hybridization need to be covered.