Structure 1.3 Electron configurations HL
Structure 1.3.6
Understandings:
Understandings:
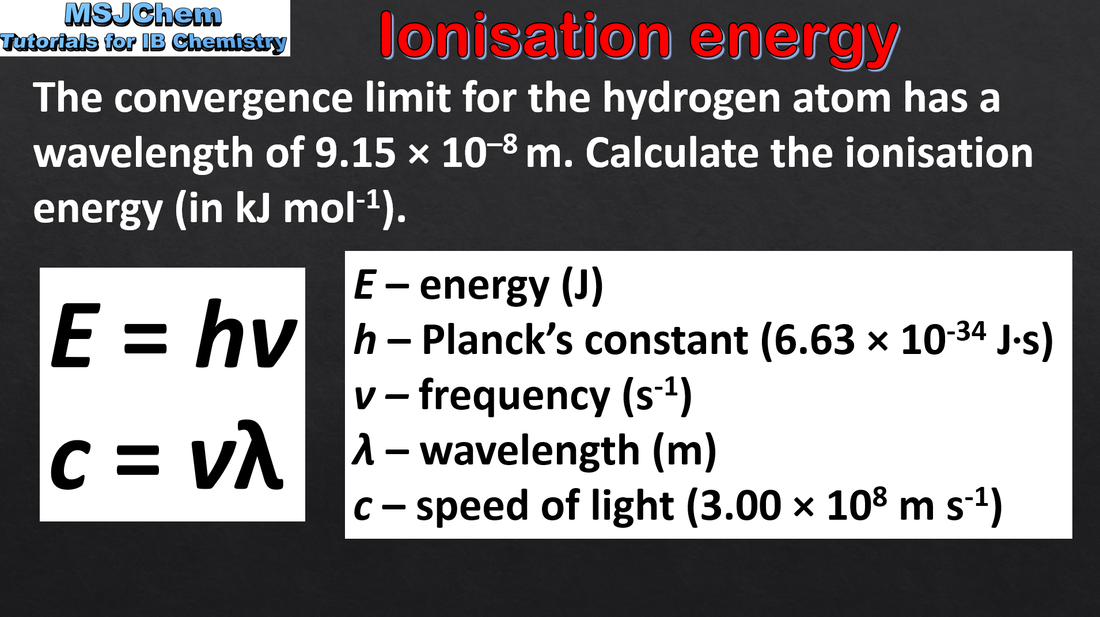
- In an emission spectrum, the limit of convergence at higher frequency corresponds to ionisation.
- Explain the trends and discontinuities in first ionisation energy (IE) across a period and down a group.
- Calculate the value of the first IE from spectral data that gives the wavelength or frequency of the convergence limit.
- The value of the Planck constant h and the equations E = hf and c = λf are given in the data booklet.
Structure 1.3.7
Understandings:
Understandings:
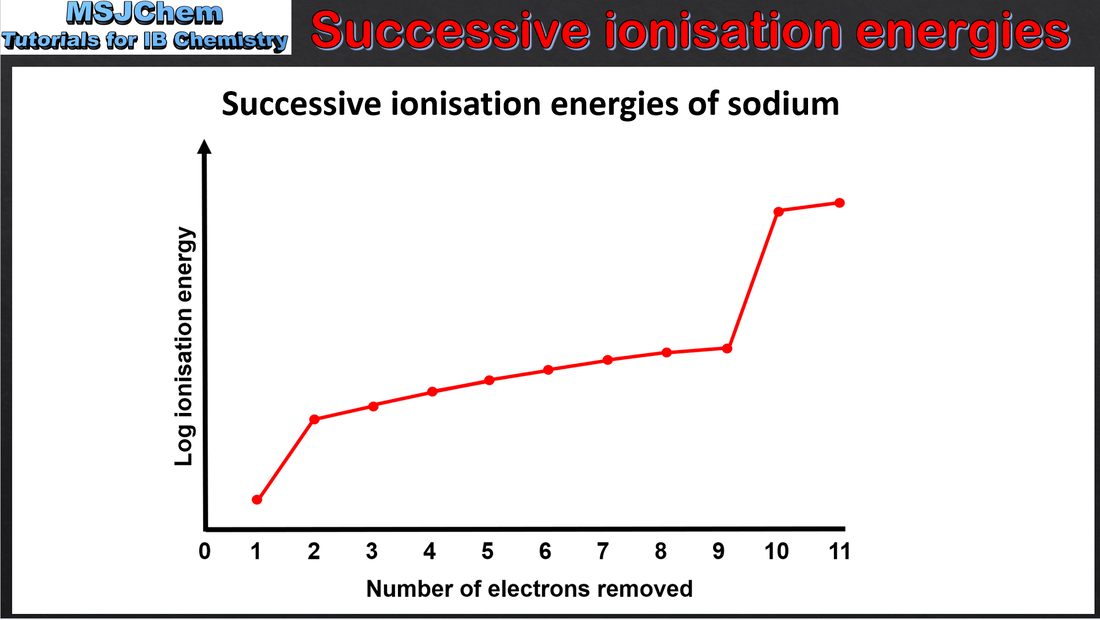
- Successive ionisation energy (IE) data for an element give information about its electron configuration.
- Deduce the group of an element from its successive ionisation data.
- Databases are useful for compiling graphs of trends in IEs.