Help support my work by joining the Member's Area or by becoming a Patron.
Topic 9 Oxidation and reduction
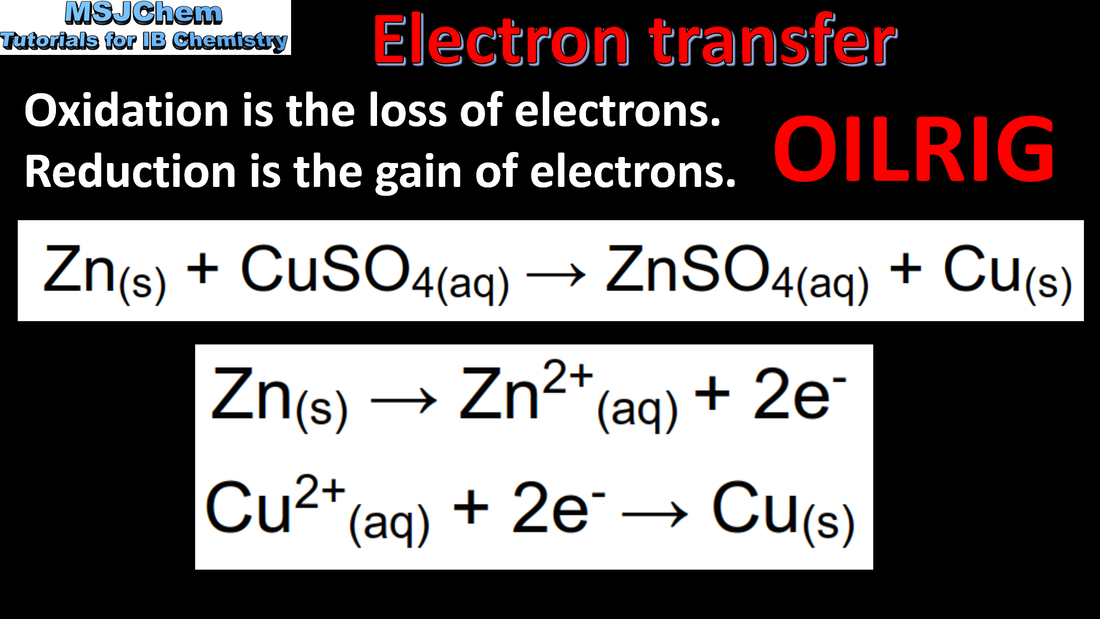
9.1 Definitions of oxidation and reduction
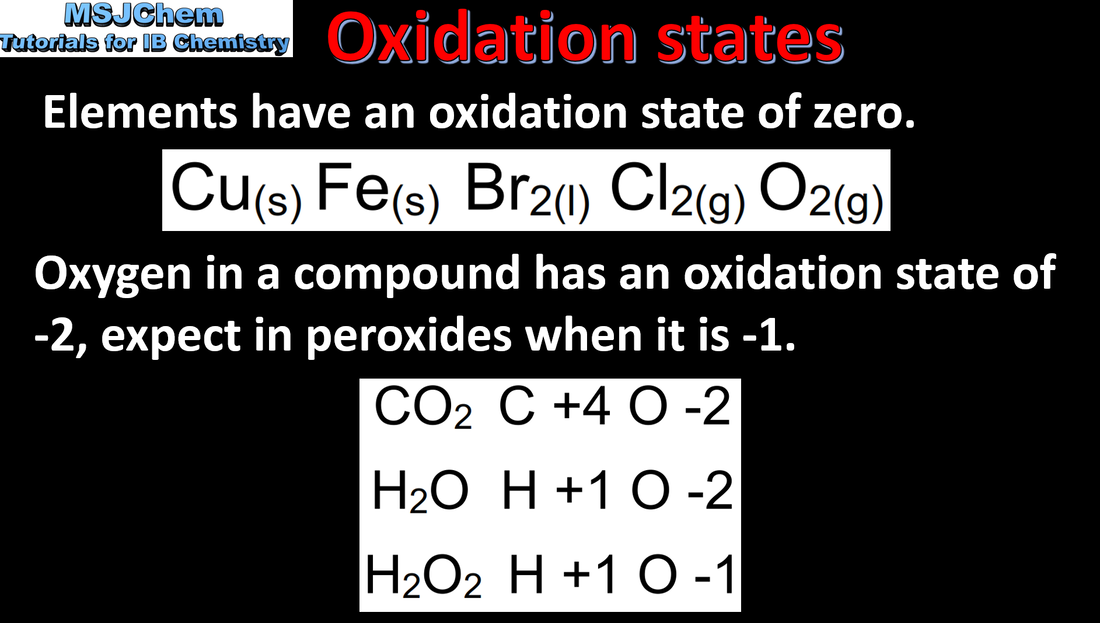
9.1 Oxidation states
|
Applications and skills:
Deduction of the oxidation states of an atom in an ion or a compound. Deduction of the name of a transition metal compound from a given formula, applying oxidation numbers represented by Roman numerals. Note - Oxidation number is shown by a Roman numeral after the name or symbol of the element. |
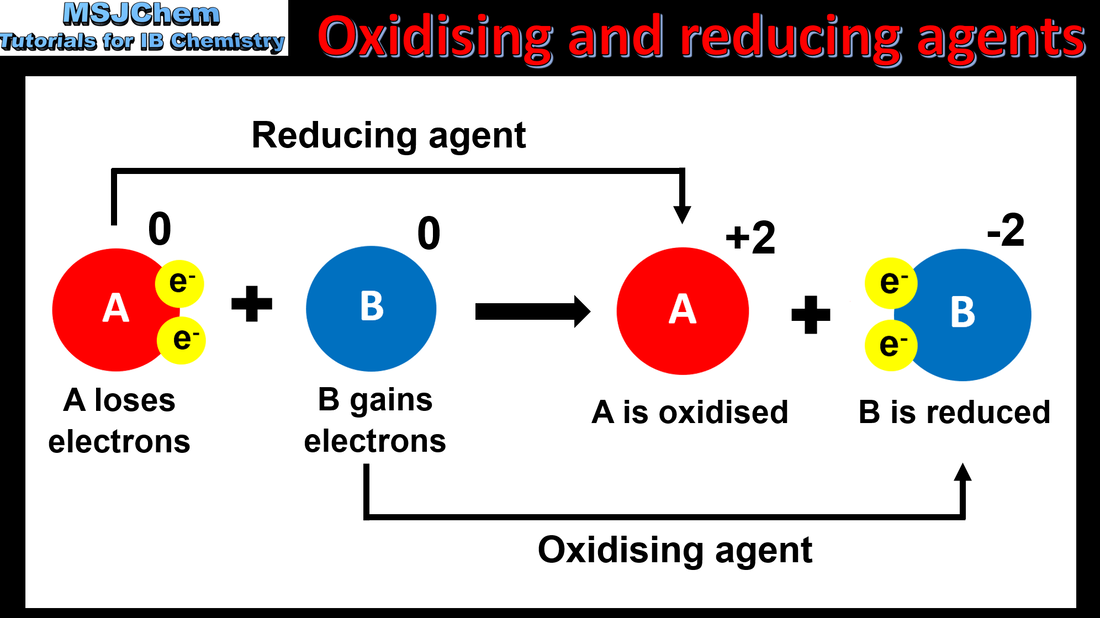
9.1 Oxidizing and reducing agents
An oxidising agent is so-called because it oxidises another species and therefore undergoes reduction. A reducing agent reduces another species and undergoes oxidation. Metals higher up in the activity series are stronger reducing agents and those lower down are weaker reducing agents. The strength of the halogens as oxidising agents decreases as you go down the group; fluorine is the strongest oxidising agent in group 17 and iodine is the weakest.
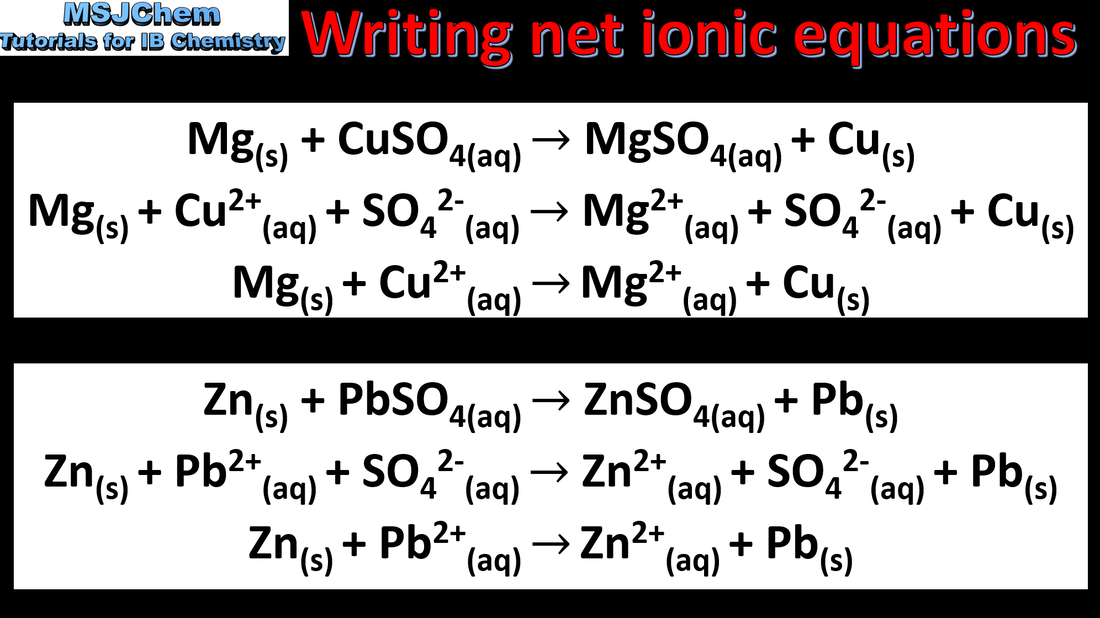
9.1 Writing net ionic equations
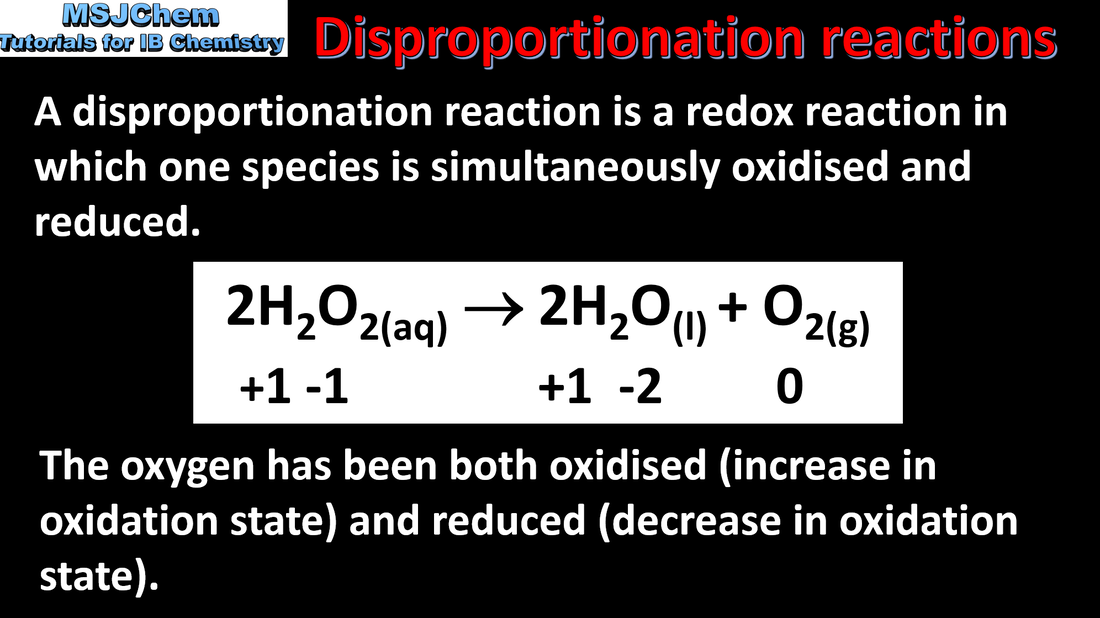
9.1 Disproportionation reactions
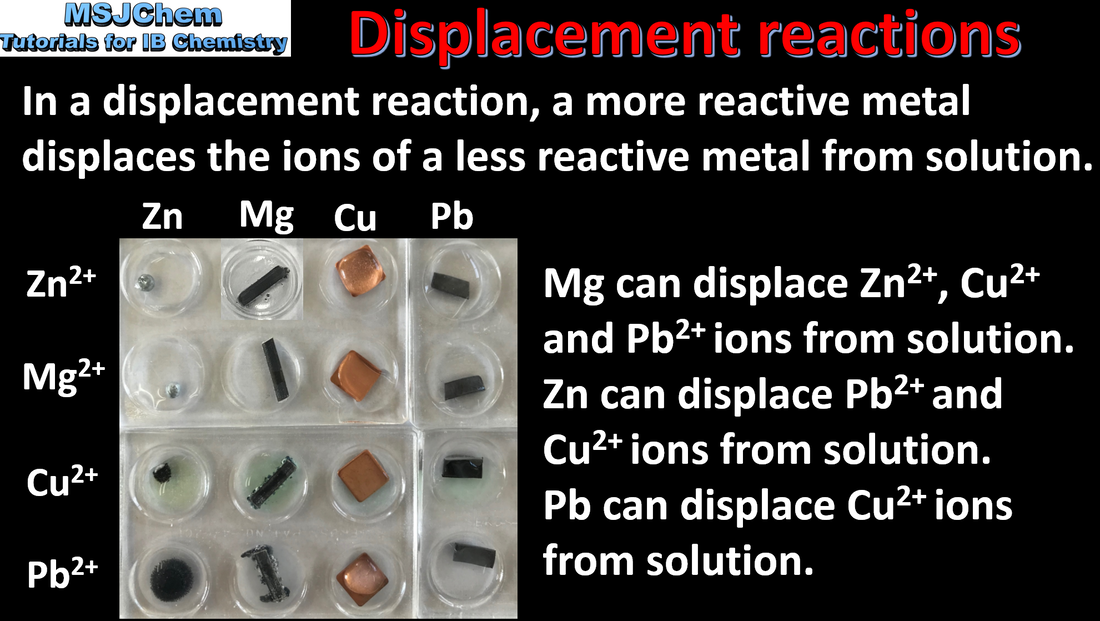
9.1 The activity series
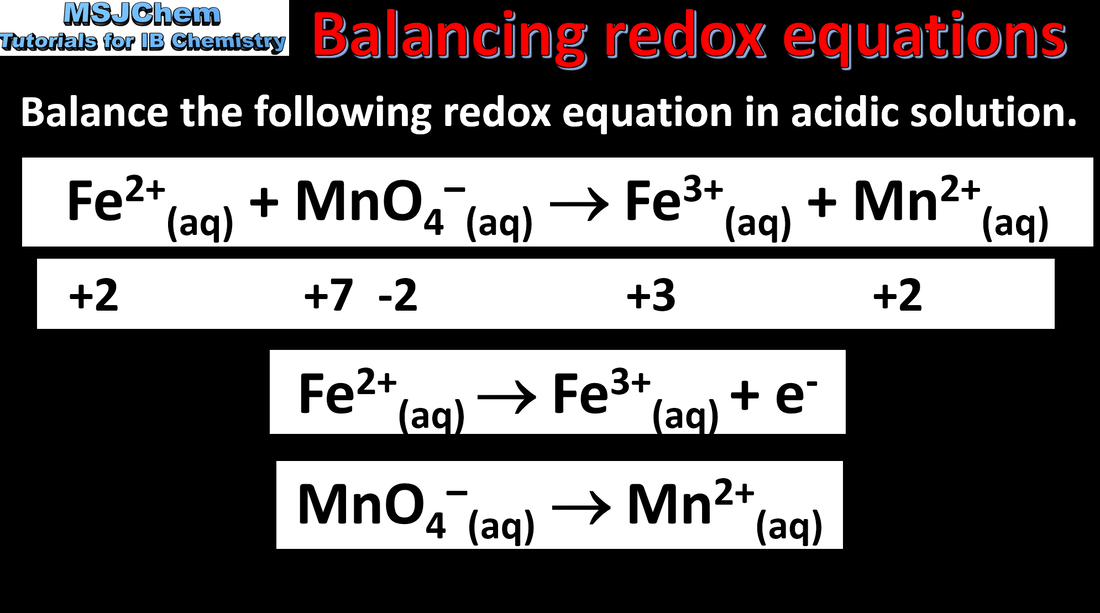
9.1 Balancing redox equations in acidic solutions
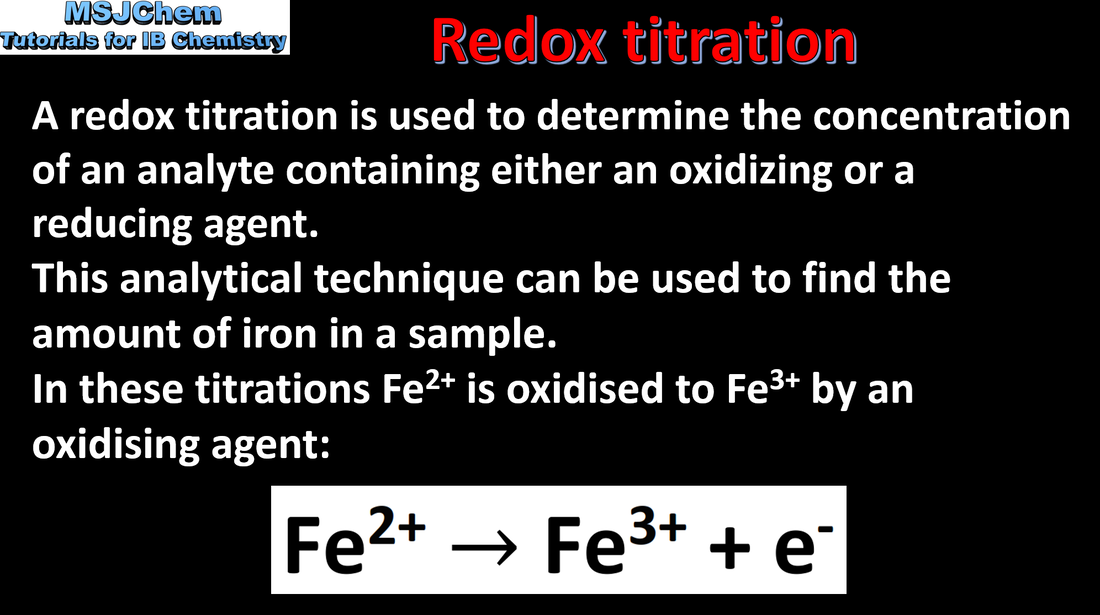
9.1 Redox titration
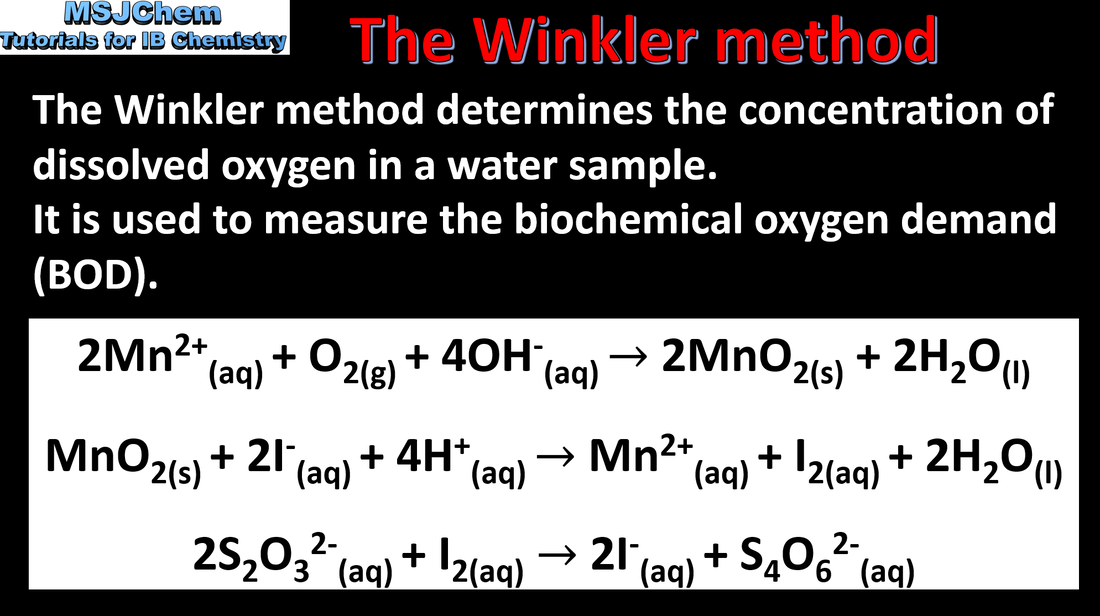
9.1 The Winkler method
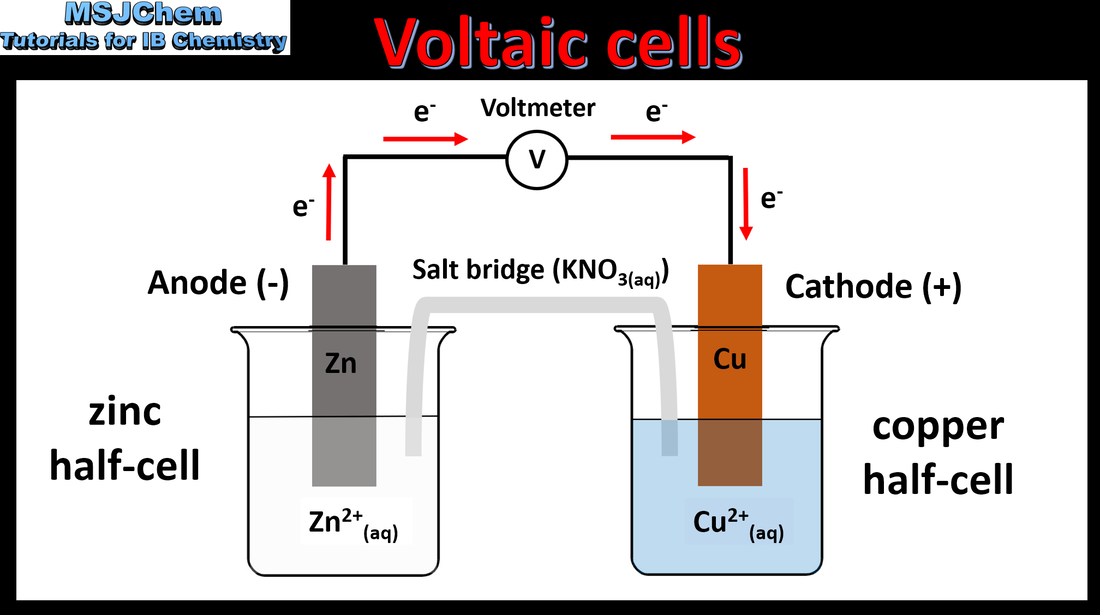
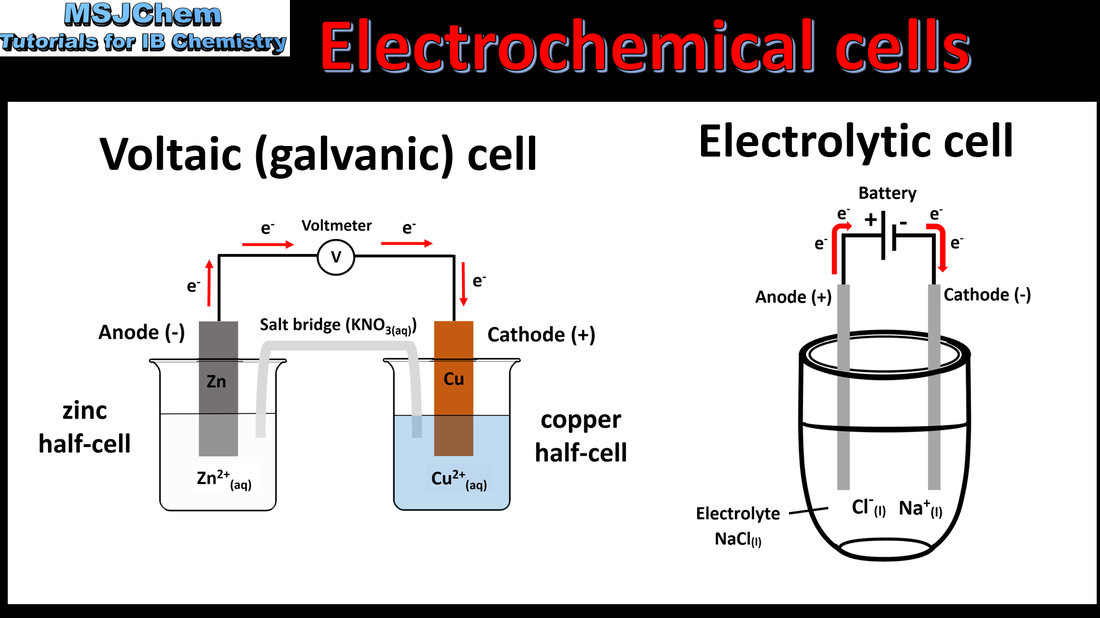
9.2 Voltaic cells
|
Understandings:
Oxidation occurs at the anode (negative electrode) and reduction occurs at the cathode (positive electrode) in a voltaic cell. Applications and skills: Construction and annotation of both types of electrochemical cells. Explanation of how a redox reaction is used to produce electricity in a voltaic cell. Distinction between electron and ion flow in voltaic cells. |
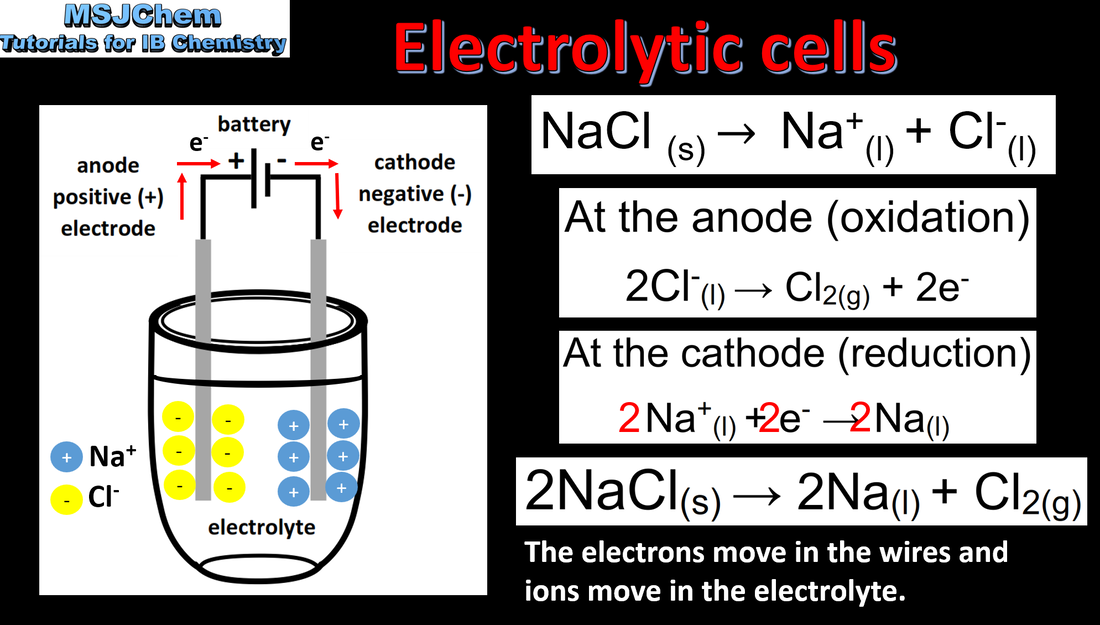
9.2 Electrolytic cells
|
Understandings:
Oxidation occurs at the anode (positive electrode) and reduction occurs at the cathode (negative electrode) in an electrolytic cell. Applications and skills Construction and annotation of both types of electrochemical cells. Explanation of how current is conducted in an electrolytic cell. Distinction between electron and ion flow in both electrochemical cells. Deduction of the products of the electrolysis of a molten salt. |
9.2 Comparison of electrochemical cells
|
Understandings:
Voltaic (Galvanic) cells: Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy. Oxidation occurs at the anode (negative electrode) and reduction occurs at the cathode (positive electrode) in a voltaic cell. Electrolytic cells: Electrolytic cells convert electrical energy to chemical energy, by bringing about non-spontaneous processes. Oxidation occurs at the anode (positive electrode) and reduction occurs at the cathode (negative electrode) in an electrolytic cells. |