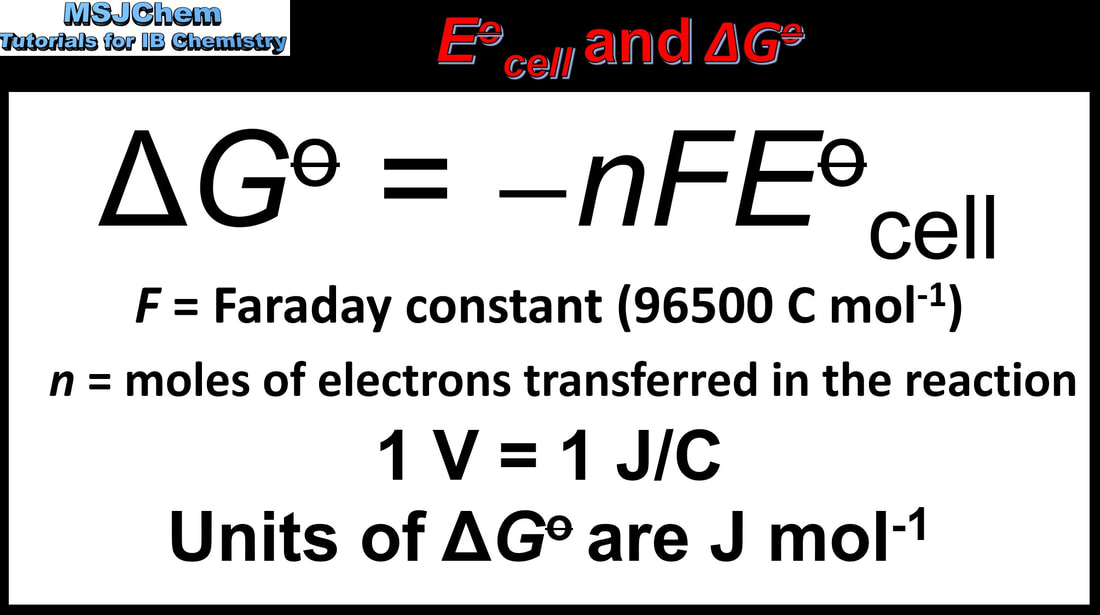Reactivity 3.2 Electron transfer reactions (HL)
Reactivity 3.2.12
Understandings:
Understandings:
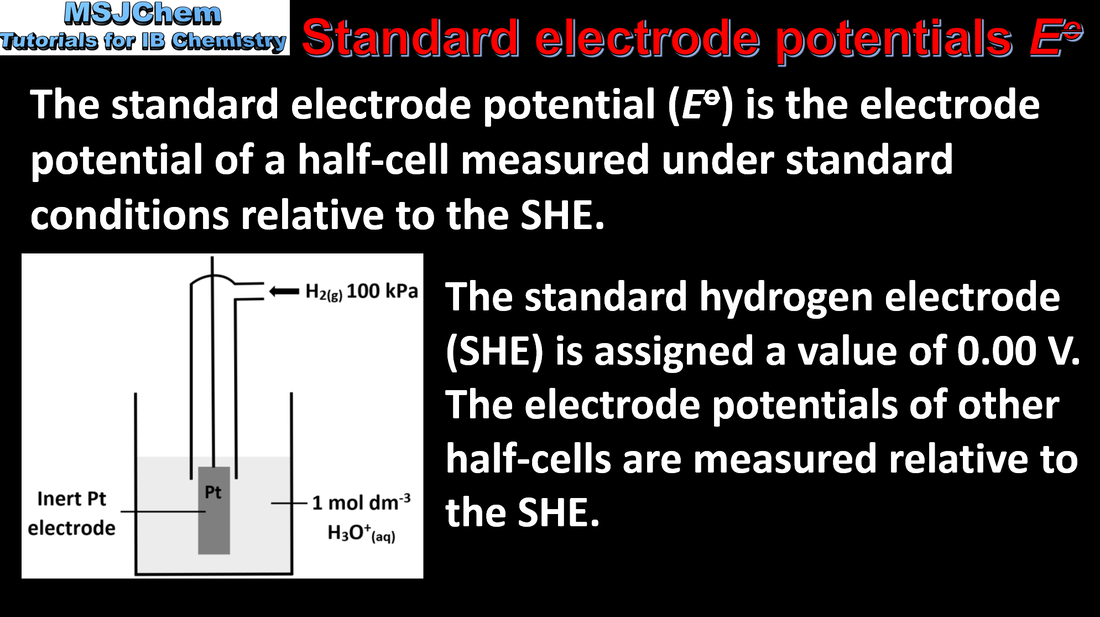
- The hydrogen half-cell H2(g) ⇌ 2H+(aq) + 2e– is assigned a standard electrode potential of zero by convention. It is used in the measurement of standard electrode potential, E⦵.
- Interpret standard electrode potential data in terms of ease of oxidation/reduction.
- Standard electrode potentials are given in the data booklet.
Reactivity 3.2.13
Understandings:
Understandings:
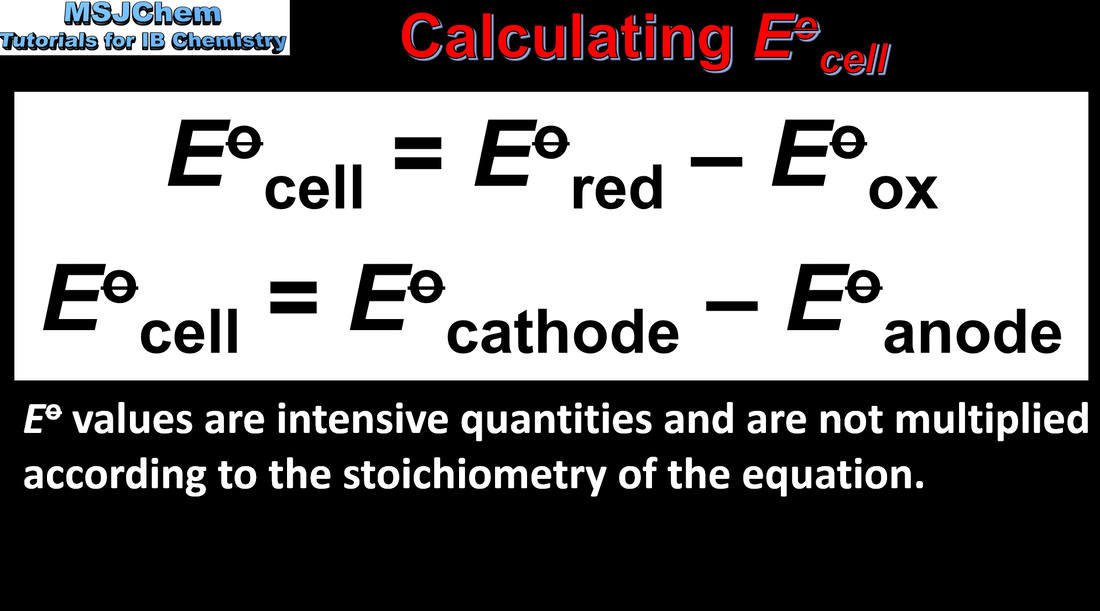
- Standard cell potential, E⦵ , can be calculated from standard potentials. E⦵ has a positive value for a spontaneous
- Predict whether a reaction is spontaneous in the forward or reverse direction from E⦵.
Reactivity 3.2.14
Understandings:
Understandings:
- The equation ΔG⦵ = − nFE⦵ shows the relationship between standard cell change in Gibbs energy and standard electrode potential for a reaction.
- Determine the value for ΔG⦵ from E⦵ data.
- The equation and the value of F in C mol–1 are given in the data booklet.
- Reactivity 1.4 How can thermodynamic data also be used to predict the spontaneity of a reaction?
Reactivity 3.2.15
Understandings:
Understandings:
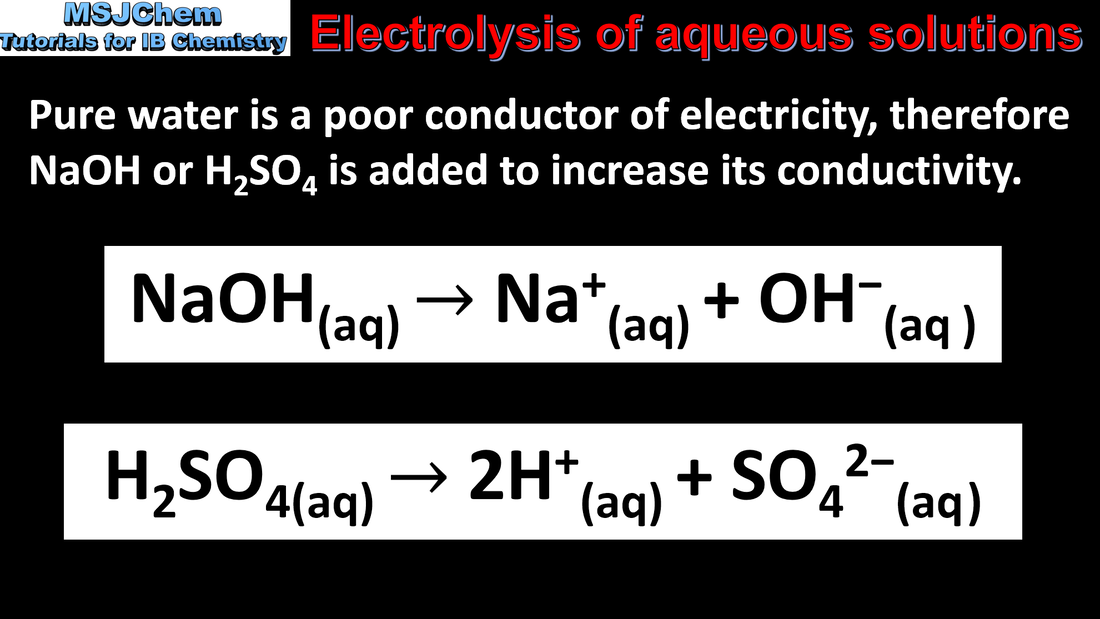
- During electrolysis of aqueous solutions, competing reactions can occur at the anode and cathode, including the oxidation and reduction of water.
- Deduce from standard electrode potentials the products of the electrolysis of aqueous solutions.
- Electrolytic processes should include the electrolysis of water and of aqueous solutions.
Reactivity 3.2.16
Understandings:
Understandings:
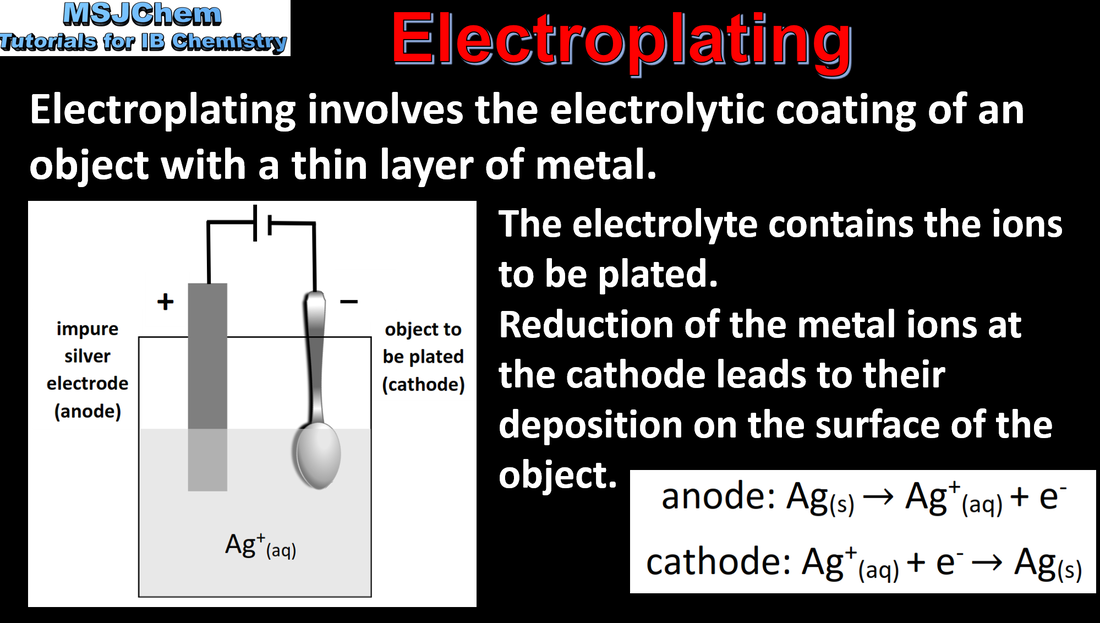
- Electroplating involves the electrolytic coating of an object with a metallic thin layer.
- Deduce equations for the electrode reactions during electroplating.