Structure 1.5 Ideal gases
Structure 1.5.1 and 1.5.2
Understandings:
Understandings:
- An ideal gas consists of moving particles with negligible volume and no intermolecular forces. All collisions between particles are considered elastic.
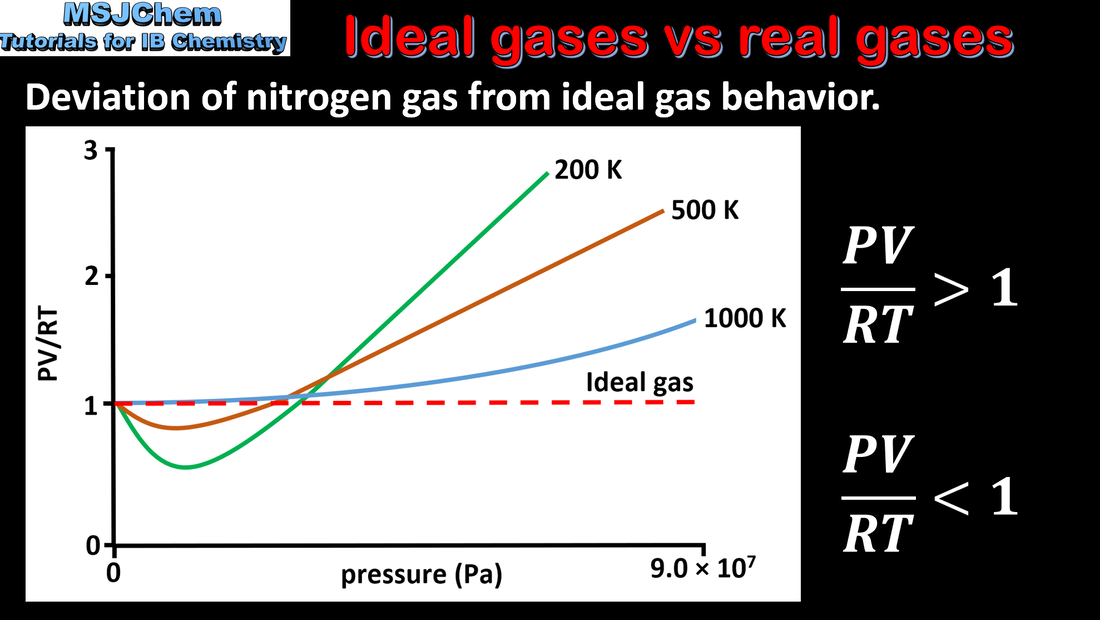
- Real gases deviate from the ideal gas model, particularly at low temperature and high pressure.
- Recognize the key assumptions in the ideal gas model.
- Explain the limitations of the ideal gas model.
Structure 1.5.3
Understandings:
Understandings:
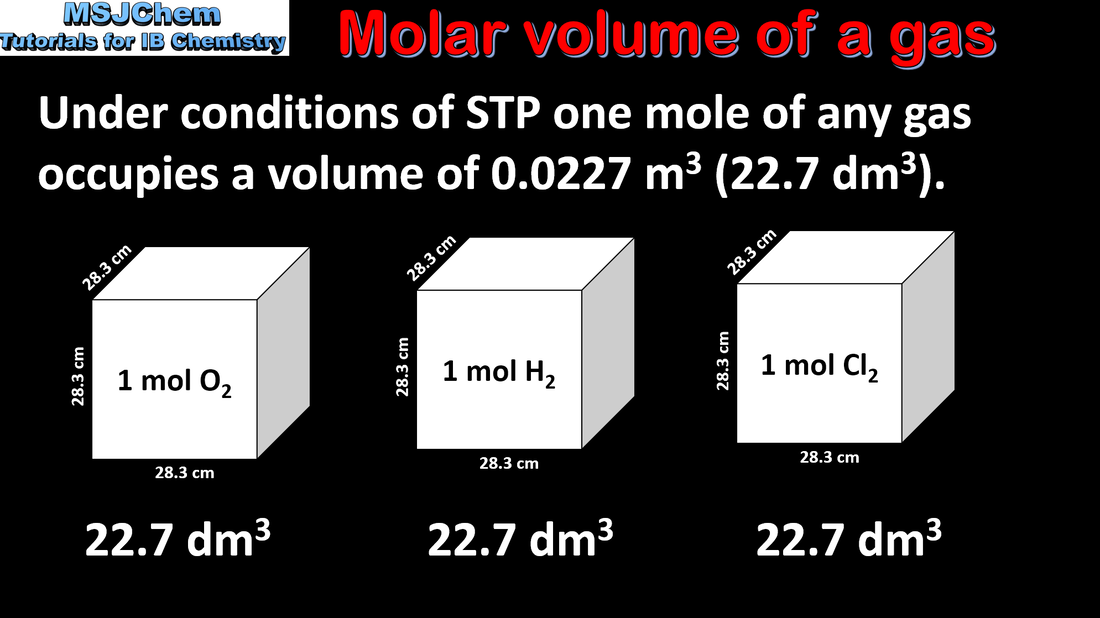
- The molar volume of an ideal gas is a constant at a specific temperature and pressure.
- Investigate the relationship between temperature, pressure and volume for a fixed mass of an ideal gas and analyse graphs relating these variables.
- The names of specific gas laws will not be assessed.
- The value for the molar volume of an ideal gas under standard temperature and pressure (STP) is given in the data booklet.
- Reactivity 2.2 Graphs can be presented as sketches or as accurately plotted data points. What are the advantages and limitations of each representation?
Structure 1.5.4
Understandings:
Understandings:
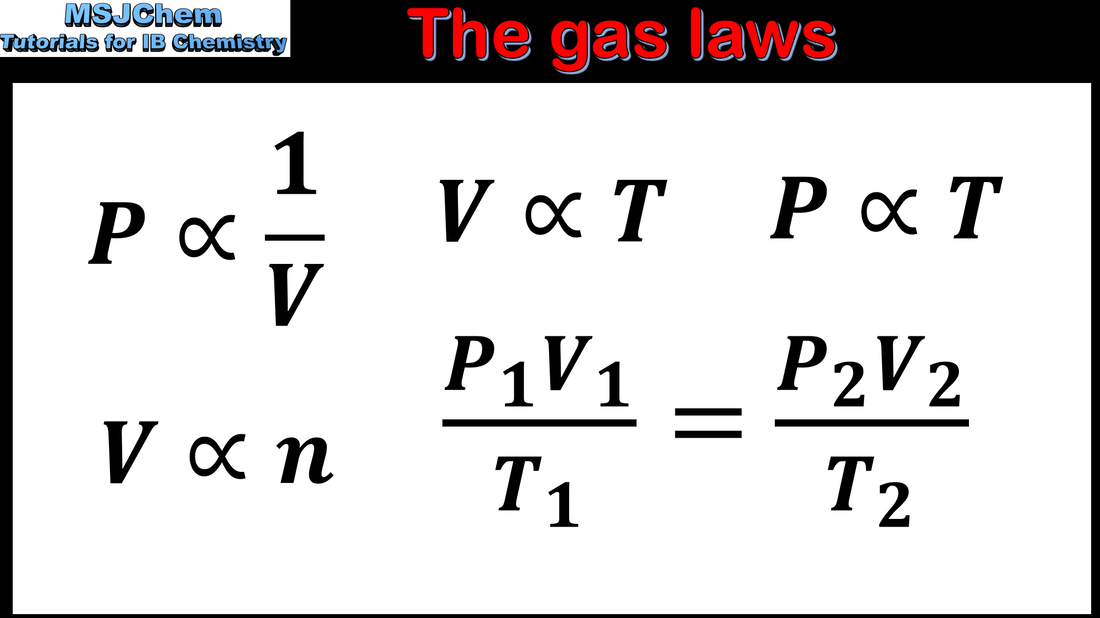
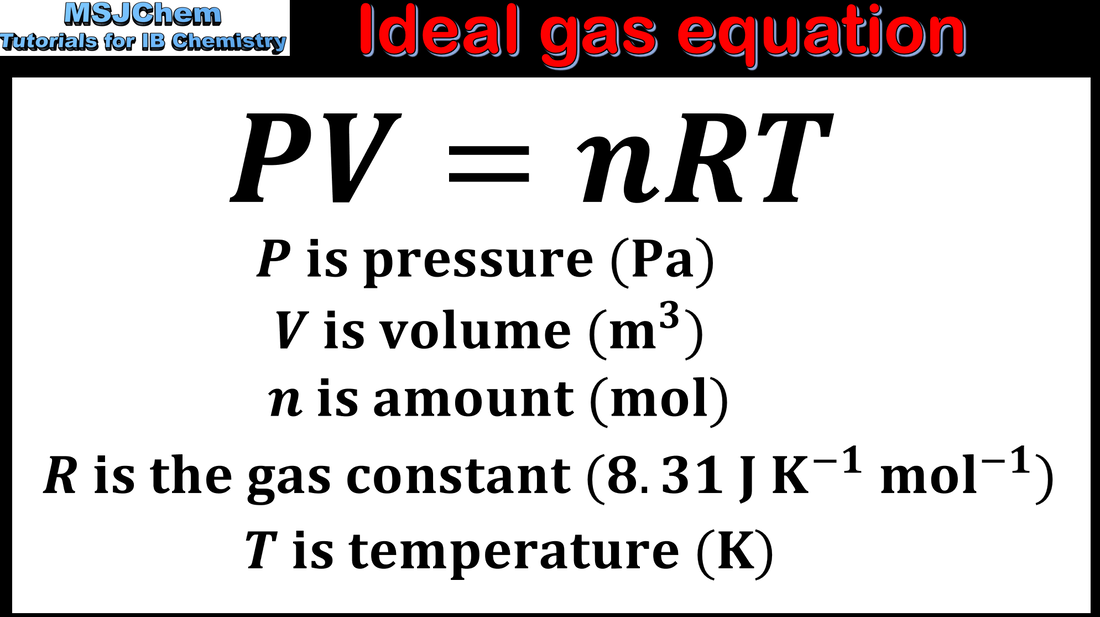
- The relationship between the pressure, volume, temperature and amount of an ideal gas is shown in the ideal gas equation PV = nRT and the combined gas law.
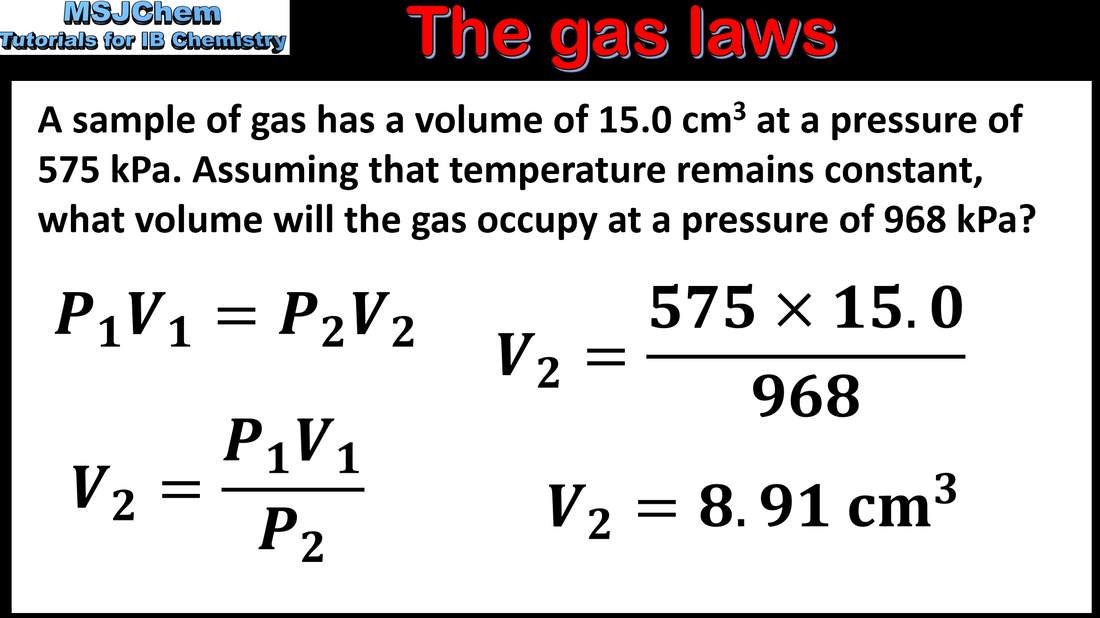
- Solve problems relating to the ideal gas equation.
- Units of volume and pressure should be SI only. The value of the gas constant R, the ideal gas equation, and the combined gas law, are given in the data booklet.