Reactivity 3.4 Electron-pair sharing reactions (HL)
Reactivity 3.4.6 and 3.4.7
Understandings:
Understandings:
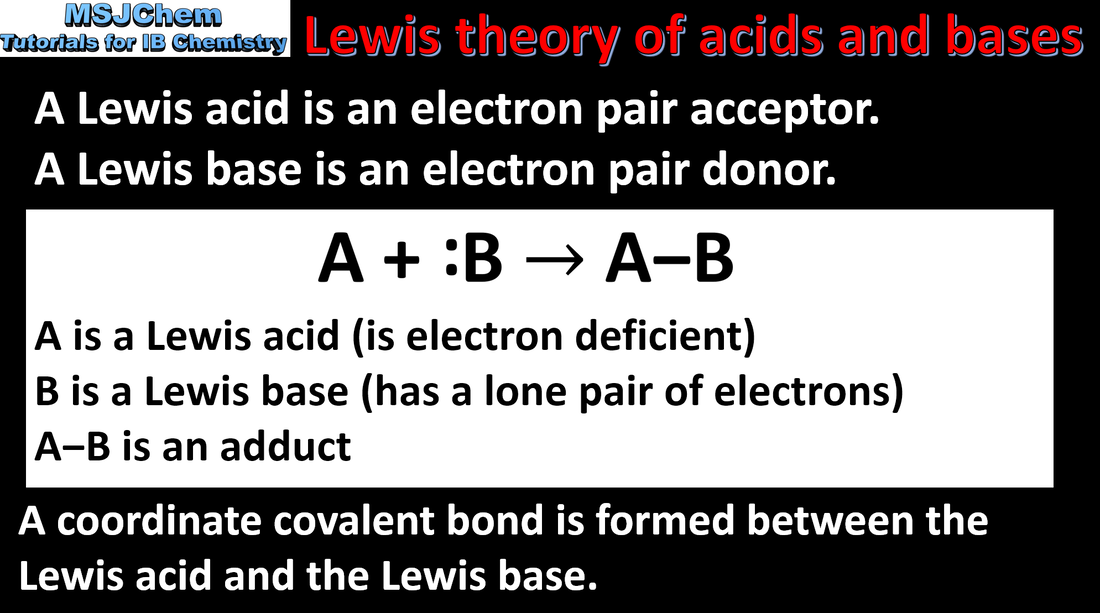
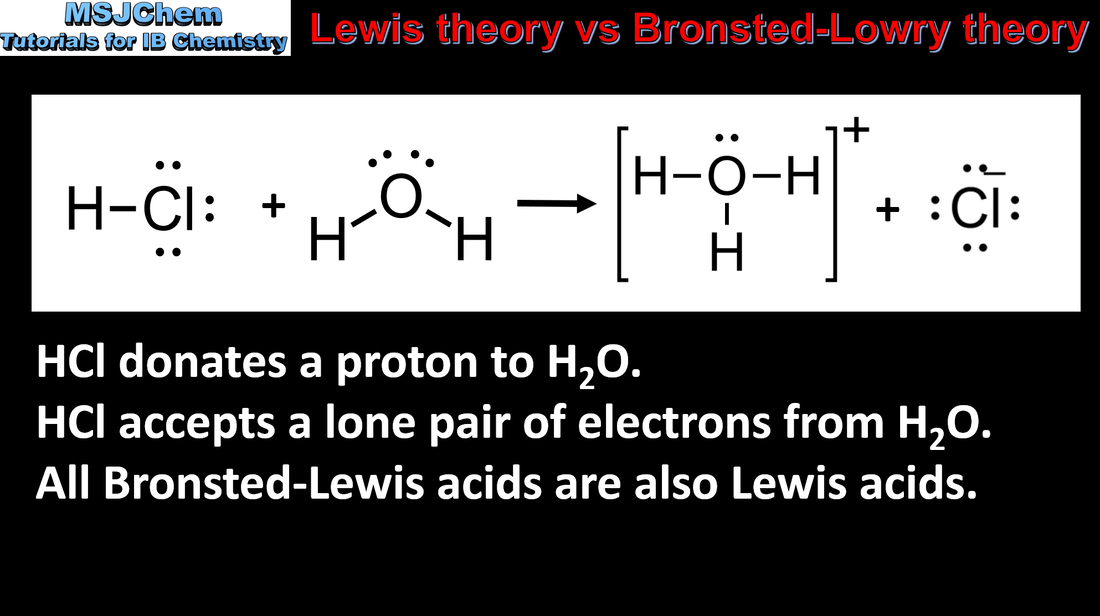
- A Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor (3.4.6).
- When a Lewis base reacts with a Lewis acid, a coordination bond is formed. Nucleophiles are Lewis bases and electrophiles are Lewis acids (3.4.7).
- Apply Lewis acid–base theory to inorganic and organic chemistry to identify the role of the reacting species (3.4.6)
- Draw and interpret Lewis formulas of reactants and products to show coordination bond formation in Lewis acid–base reactions (3.4.7).
- Reactivity 3.1 What is the relationship between Brønsted–Lowry acids and bases and Lewis acids and bases?
- Structure 2.2 Do coordination bonds have any different properties from other covalent bonds?
Reactivity 3.4.8
Understandings:
Understandings:
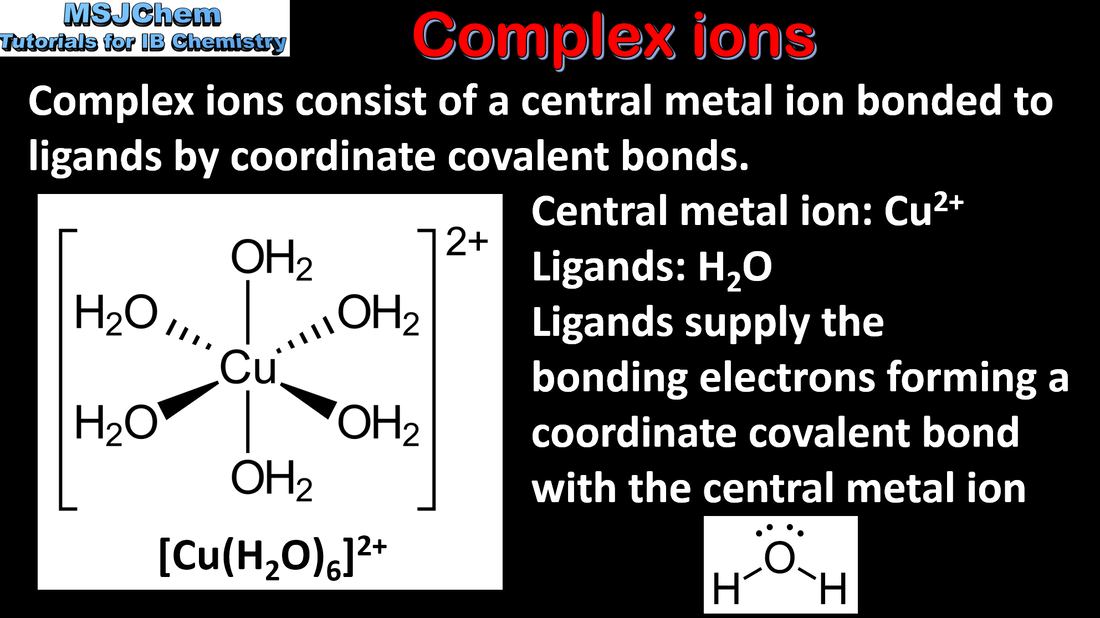
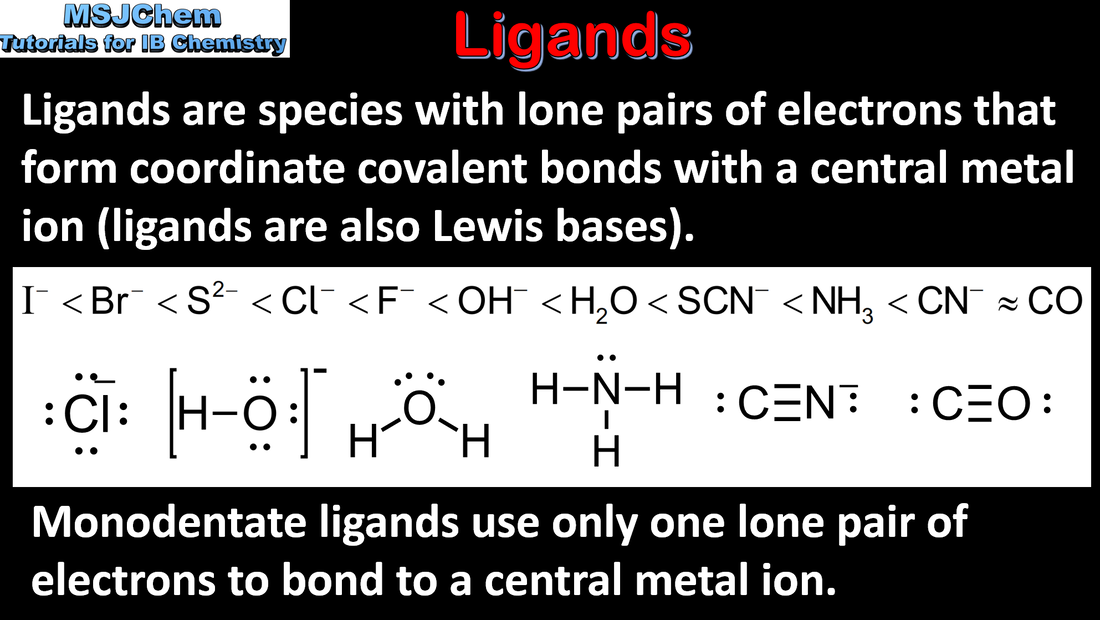
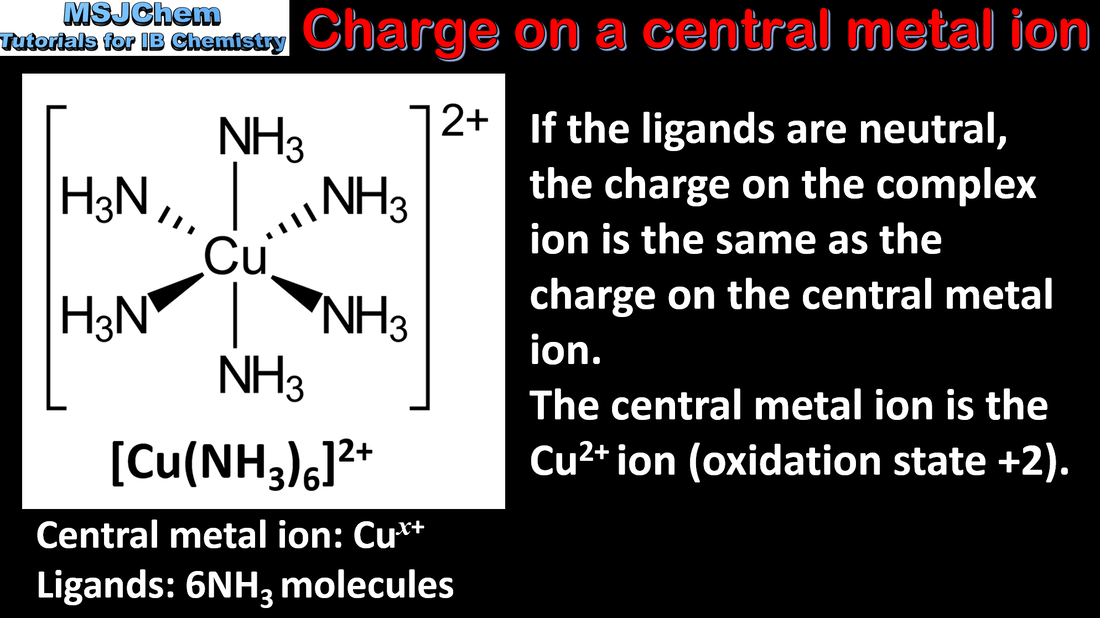
- Coordination bonds are formed when ligands donate an electron pair to transition element cations, forming complex ions.
- Deduce the charge on a complex ion, given the formula of the ion and ligands present.
Reactivity 3.4.9
Understandings:
Understandings:
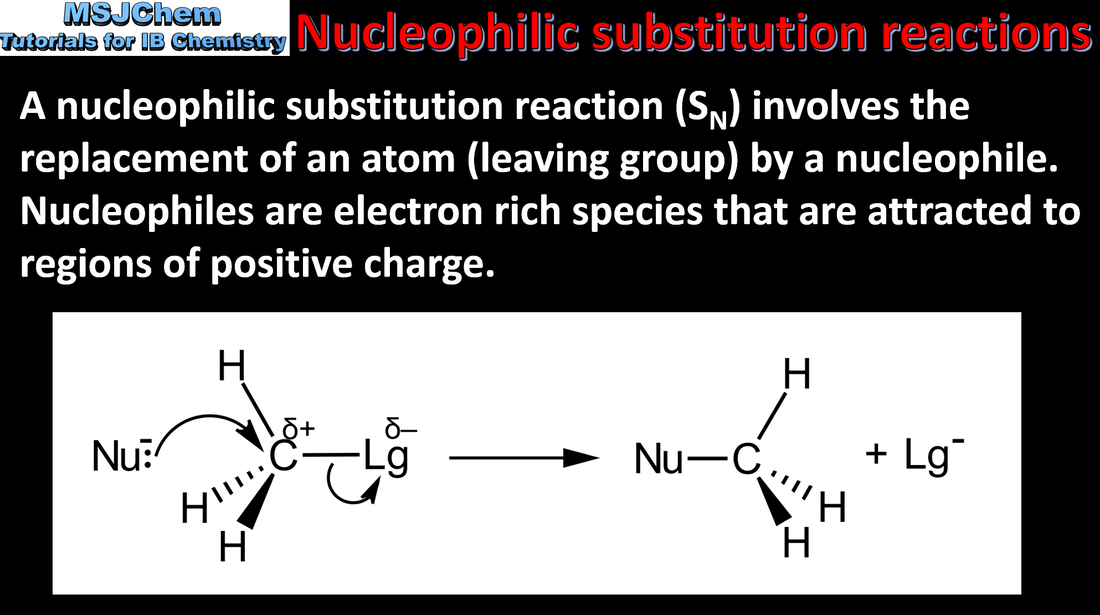
- Nucleophilic substitution reactions include the reactions between halogenoalkanes and nucleophiles.
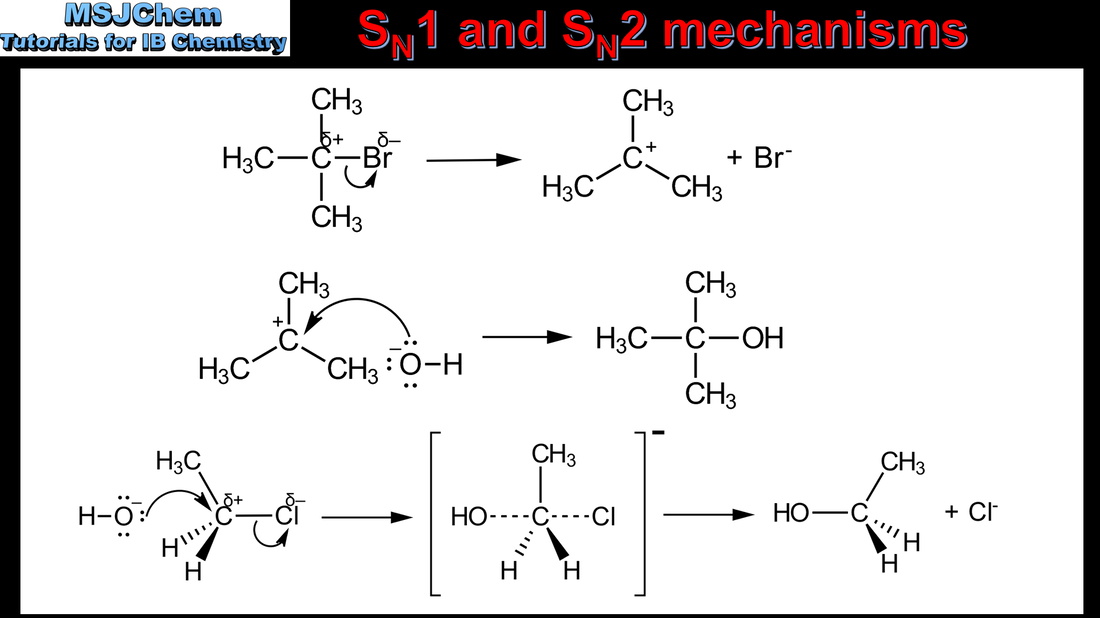
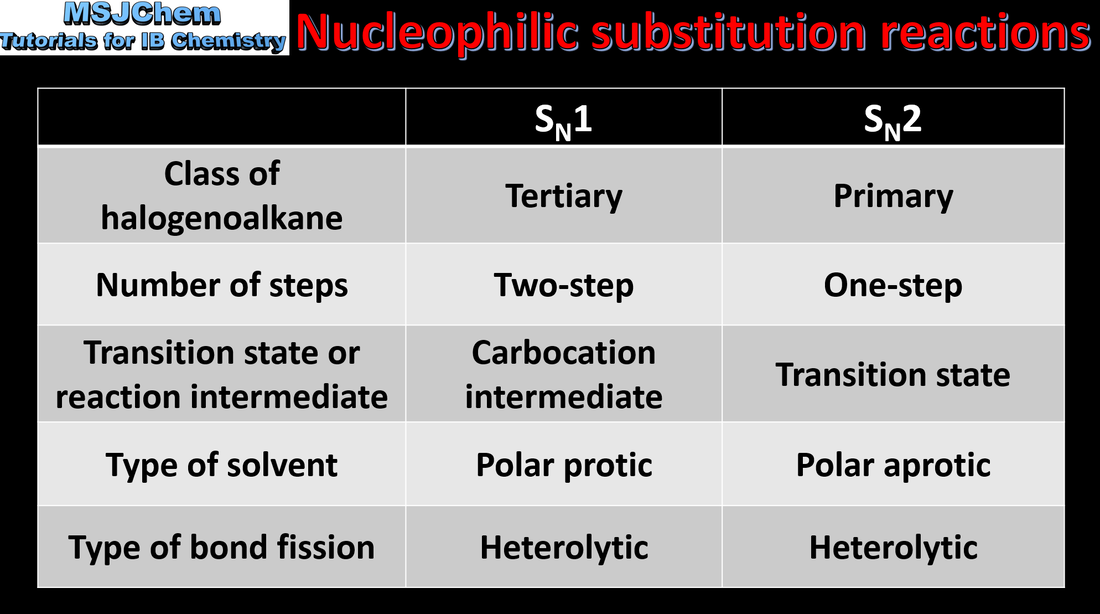
- Describe and explain the mechanisms of the reactions of primary and tertiary halogenoalkanes with nucleophiles.
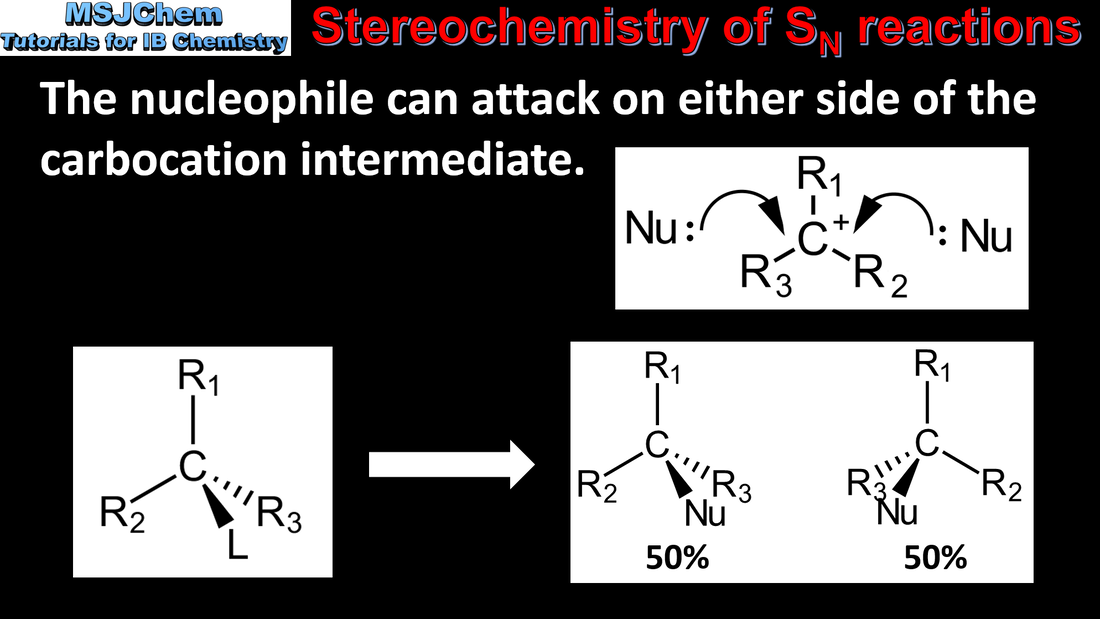
- Distinguish between the concerted one-step SN2 reaction of primary halogenoalkanes and the two-step SN1 reaction of tertiary halogenoalkanes. Both mechanisms occur for secondary halogenoalkanes.
- The stereospecific nature of SN2 reactions should be covered.
- Reactivity 2.2 What differences would be expected between the energy profiles for SN1 and SN2 reactions?
- Reactivity 2.2 What are the rate equations for these SN1 and SN2 reactions?
Reactivity 3.4.10
Understandings:
Understandings:
- The rate of the substitution reactions is influenced by the identity of the leaving group.
- Predict and explain the relative rates of the substitution reactions for different halogenoalkanes.
- Different halogenoalkanes should include RCl, RBr and RI.
- The roles of the solvent and the reaction mechanism on the rate will not be assessed.
- Structure 3.1 Why is the iodide ion a better leaving group than the chloride ion?
|
Video coming soon.
|
Reactivity 3.4.11
Understandings:
Understandings:
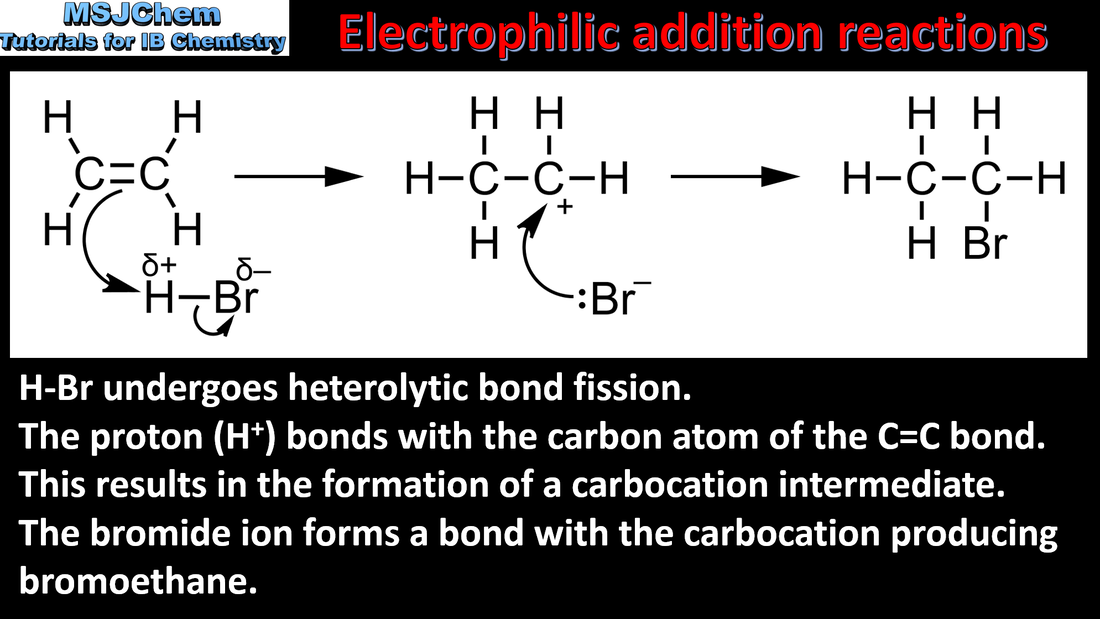
- Alkenes readily undergo electrophilic addition reactions.
- Describe and explain the mechanisms of the reactions between symmetrical alkenes and halogens, water and hydrogen halides.
Reactivity 3.4.12
Understandings:
Understandings:
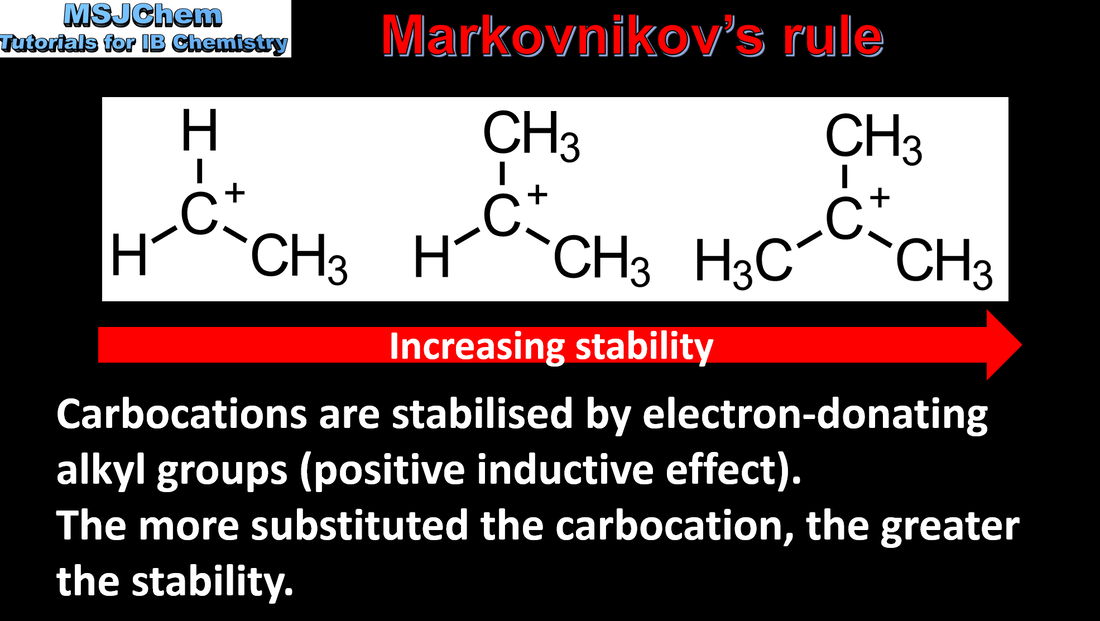
- The relative stability of carbocations in the addition reactions between hydrogen halides and unsymmetrical alkenes can be used to explain the reaction mechanism.
- Predict and explain the major product of a reaction between an unsymmetrical alkene and a
hydrogen halide or water.
Reactivity 3.4.13
Understandings:
Understandings:
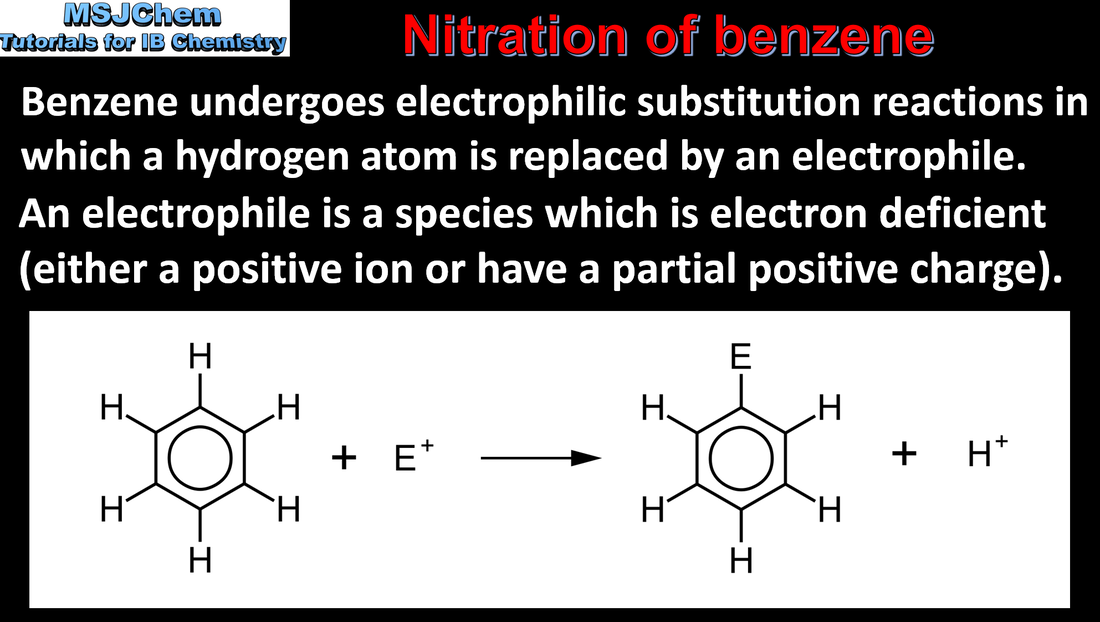
- Electrophilic substitution reactions include the reactions of benzene with electrophiles.
- Describe and explain the mechanism of the reaction between benzene and a charged electrophile, E+.
- The formation of the electrophile will not be assessed.
- Structure 2.2 What are the features of benzene, C6H6, that make it not prone to undergo addition reactions, despite being highly unsaturated?
- Reactivity 3.1 Nitration of benzene uses a mixture of concentrated nitric and sulfuric acids to generate a strong electrophile, NO2 . How can the acid/base behaviour of HNO3 in this mixture be described?