Topic 12 Atomic structure HL
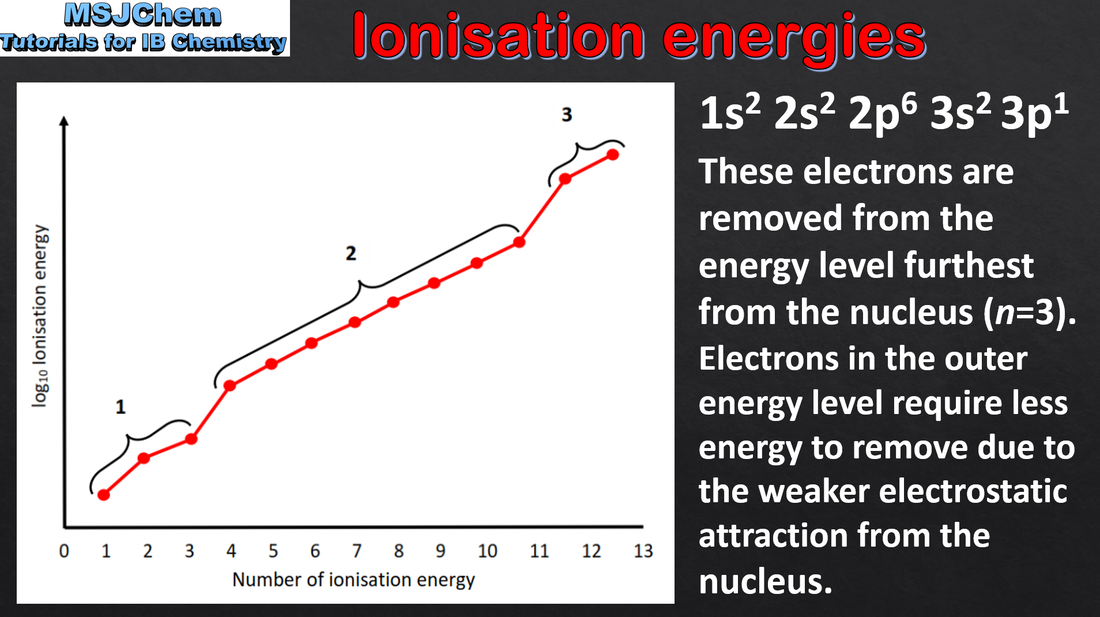
12.1 Successive ionisation energies
|
Understandings:
Trends in first ionization energy across periods account for the existence of main energy levels and sub-levels in atoms. Successive ionization energy data for an element give information that shows relations to electron configurations. Applications and skills: Deduction of the group of an element from its successive ionization energy data. Explanation of the trends and discontinuities in first ionization energy across a period. |
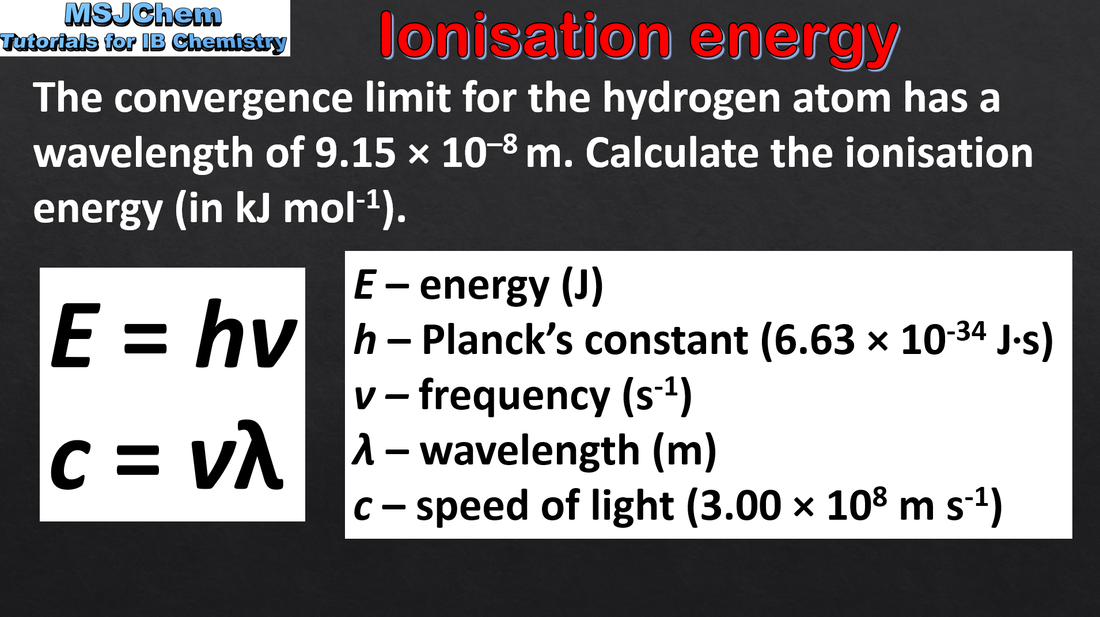
12.1 Calculating ionisation energy
|
Understandings:
In an emission spectrum, the limit of convergence at higher frequency corresponds to the first ionization energy. Applications and skills: Solving problems using E=hv. Calculation of the value of the first ionization energy from spectral data which gives the wavelength or frequency of the convergence limit. |