Option C Energy SL
YouTube Playlist
YouTube Playlist
Help support me to keep making these videos by clicking on the link below.
C.1 Introduction to energy
|
|
Understandings:
A useful energy source releases energy at a reasonable rate and produces minimal pollution. The quality of energy is degraded as heat is transferred to the surroundings. Energy and materials go from a concentrated into a dispersed form. The quantity of the energy available for doing work decreases. |
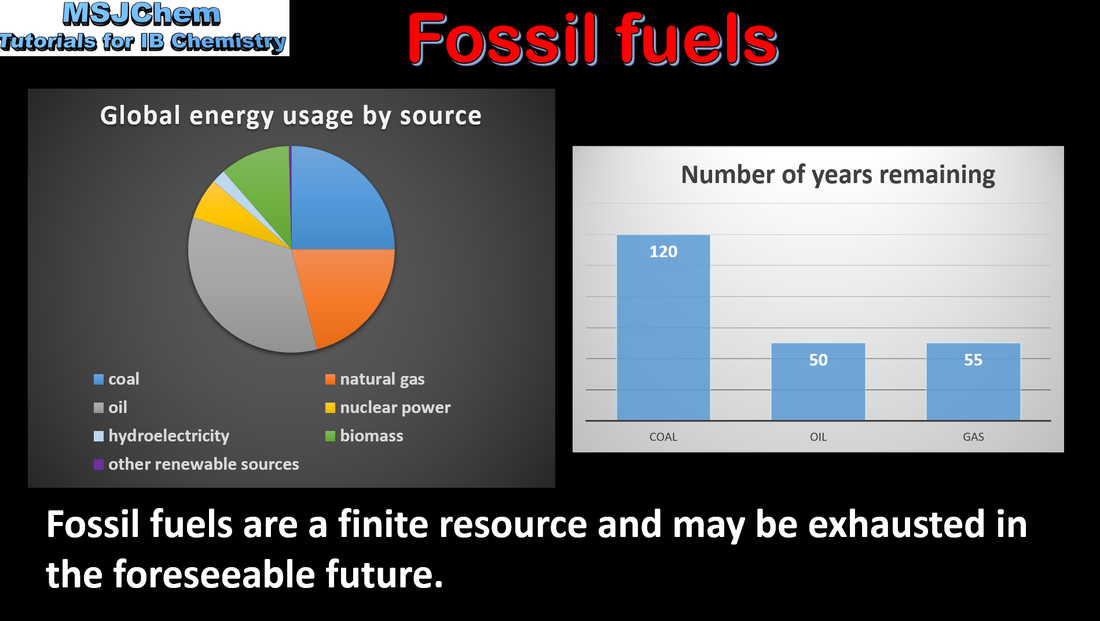
C.1 Renewable and non-renewable energy sources
|
|
Understandings:
Renewable energy sources are naturally replenished. Non-renewable energy sources are finite. Applications and skills: Discussion of the use of different sources of renewable and non-renewable energy. |
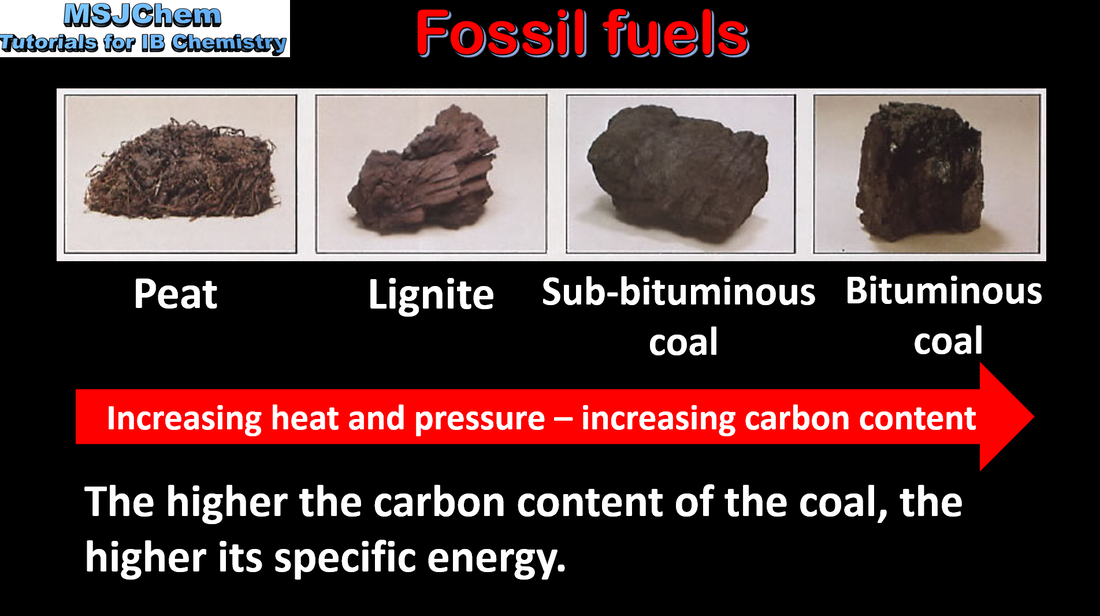
C.1 Specific energy and energy density
|
|
Applications and skills:
Determination of the energy density and specific energy of a fuel from the enthalpies of combustion, densities and the molar mass of fuel. Discussion of how the choice of fuel is influenced by its energy density or specific energy. |
C.1 Efficiency of energy transfers
|
|
Applications and skills:
Determination of the efficiency of an energy transfer process from appropriate data. |
C.2 Fractional distillation of crude oil
|
|
Understandings:
Petroleum is a complex mixture of hydrocarbons that can be split into different component parts called fractions by fractional distillation. Crude oil needs to be refined before use. The different fractions are separated by a physical process in fractional distillation. Applications and skills: Identification of the various fractions of petroleum, their relative volatility and their uses. |
C.2 Thermal and catalytic cracking
|
|
Understandings:
The performance of hydrocarbons as fuels is improved by the cracking reactions. Applications and skills: Deduction of equations for cracking reactions. |
C.2 Octane number
|
|
Understandings:
The tendency of a fuel to auto-ignite, which leads to “knocking” in a car engine, is related to molecular structure and measured by the octane number. Applications and skills: Discussion of the reforming and cracking reactions of hydrocarbons and explanation how these processes improve the octane number. |
C.2 Catalytic reforming
|
|
Understandings:
The performance of hydrocarbons as fuels is improved by the cracking and catalytic reforming reactions. Applications and skills: Discussion of the reforming and cracking reactions of hydrocarbons and explanation how these processes improve the octane number. |
C.2 Isomerisation
|
Note that isomerisation is a type of catalytic reforming, therefore students should be able to deduce the products of reforming (isomerisation) reactions producing branched isomers.
|
Understandings:
The performance of hydrocarbons as fuels is improved by the cracking and catalytic reforming reactions. Applications and skills: Discussion of the effect of chain length and chain branching on the octane number. Discussion of the reforming and cracking reactions of hydrocarbons and explanation how these processes improve the octane number. |
C.2 Coal gasification and liquefaction
|
|
Understandings:
Coal gasification and liquefaction are chemical processes that convert coal to gaseous and liquid hydrocarbons. Applications and skills: Deduction of equations for cracking and reforming reactions, coal gasification and liquefaction. |
C.2 Carbon footprint
|
|
Understandings:
A carbon footprint is the total amount of greenhouse gases produced during human activities. It is generally expressed in equivalent tons of carbon dioxide Applications and skills: Calculations of the carbon dioxide added to the atmosphere, when different fuels burn and determination of carbon footprints for different activities. |
C.3 Uses of nuclear fusion and nuclear fission
|
|
Understandings:
Fusion reactions are a promising energy source as the fuel is inexpensive and abundant, and no radioactive waste is produced. Guidance: The workings of a nuclear power plant are not required. |
C.3 Nuclear fusion
|
|
This video covers nuclear fusion.
Applications and skills: Construction of nuclear equations for fusion reactions. |
C.3 Nuclear fission
|
|
Understandings:
U-235 undergoes a fission chain reaction. The critical mass is the mass of fuel needed for the reaction to be self- sustaining. Applications and skills: Deduction of nuclear equations for fission reactions. |
C.3 Nuclear binding energy
|
|
Understandings:
Light nuclei can undergo fusion reactions as this increases the binding energy per nucleon. Heavy nuclei can undergo fission reactions as this increases the binding energy per nucleon. Applications and skills: Explanation of fusion reactions in terms of binding energy per nucleon. Explanation of fission reactions in terms of binding energy per nucleon. |
C.3 Half-life
|
|
Understandings:
Half-life is the time it takes for half the number of atoms to decay. Applications and skills: Solution of radioactive decay problems involving integral numbers of half-lives. |
C.3 Calculating the decay constant
|
|
This video covers how to calculate the decay constant for a radioactive isotope.
Note that the equation in the video is given in section 1 of the data booklet. |
C.3 Radioactive waste
|
|
Understandings:
Radioactive waste may contain isotopes with long and short half-lives. Applications and skills: Discussion of the storage and disposal of nuclear waste. |
C.3 Types of radioactive decay
|
|
This video covers the different types of radioactive decay; alpha, beta minus, beta plus and gamma decay.
|
C.3 Fast breeder reactors
|
|
Understandings:
239Pu, used as a fuel in “breeder reactors”, is produced from 238U by neutron capture. |
C.3 Absorption spectra of stars
|
|
Understandings:
Absorption spectra are used to analyse the composition of stars. Applications and skills: Explanation of the atomic absorption spectra of hydrogen and helium, including the relationships between the lines and electron transitions. |
C.4 Photosynthesis
|
|
Understandings:
Light can be absorbed by chlorophyll and other pigments with a conjugated electronic structure. Photosynthesis converts light energy into chemical energy: 6CO2 + 6H2O ==> C6H12O6 + 6O2 Applications and skills: Identification of features of the molecules that allow them to absorb visible light. Guidance: Only a conjugated system with alternating double bonds needs to be covered. |
C.4 Biofuels
|
|
Understandings:
Fermentation of glucose produces ethanol which can be used as a biofuel: C6H12O6 ==> 2C2H5OH + 2CO2 Applications and skills: Evaluation of the advantages and disadvantages of the use of biofuels. |
C.4 Transesterification
|
|
Understandings:
Transesterification between an ester and an alcohol with a strong acid or base catalyst produces a different ester: RCOOR1 + R2OH ==> RCOOR2 + R1OH In the transesterification process, involving a reaction with an alcohol in the presence of a strong acid or base, the triglyceride vegetable oils are converted to a mixture mainly comprising of alkyl esters and glycerol, but with some fatty acids. Transesterification with ethanol or methanol produces oils with lower viscosity that can be used in diesel engines. Applications and skills: Deduction of equations for transesterification reactions. |
C.5 The greenhouse effect
|
|
Understandings:
Greenhouse gases allow the passage of incoming solar short wavelength radiation but absorb the longer wavelength radiation from the Earth. Some of the absorbed radiation is re-radiated back to Earth. |
C.5 Greenhouse gases
|
|
Applications and skills:
Discussion of the sources, relative abundance and effects of different greenhouse gases. Guidance: Greenhouse gases to be considered are CH4, H2O and CO2. |
C.5 IR absorbance of greenhouse gases
|
|
Understandings:
Greenhouse gases absorb IR radiation as there is a change in dipole moment as the bonds in the molecule stretch and bend. Applications and skills: Explanation of the molecular mechanisms by which greenhouse gases absorb infrared radiation. |
C.5 Effects of global warming
|
|
This video covers the influence of increasing amounts of greenhouse gases on the atmosphere.
|
C.5 Reducing greenhouse gas emissions
|
|
Applications and skills:
Discussion of the different approaches to the control of carbon dioxide emissions. |
C.5 Evidence for global warming
|
Applications and skills:
Discussion of the evidence for the relationship between the increased concentration of gases and global warming. |
C.5 Ocean acidification
|
|
Understandings:
There is a heterogeneous equilibrium between concentration of atmospheric carbon dioxide and aqueous carbon dioxide in the oceans. Applications and skills: Discussion of pH changes in the ocean due to increased concentration of carbon dioxide in the atmosphere. |
C.5 Global dimming
|
|
Understandings:
Particulates such as smoke and dust cause global dimming as they reflect sunlight, as do clouds. |