Structure 2.4 From models to materials
Structure 2.4.1 and 2.4.2
Understandings:
Understandings:
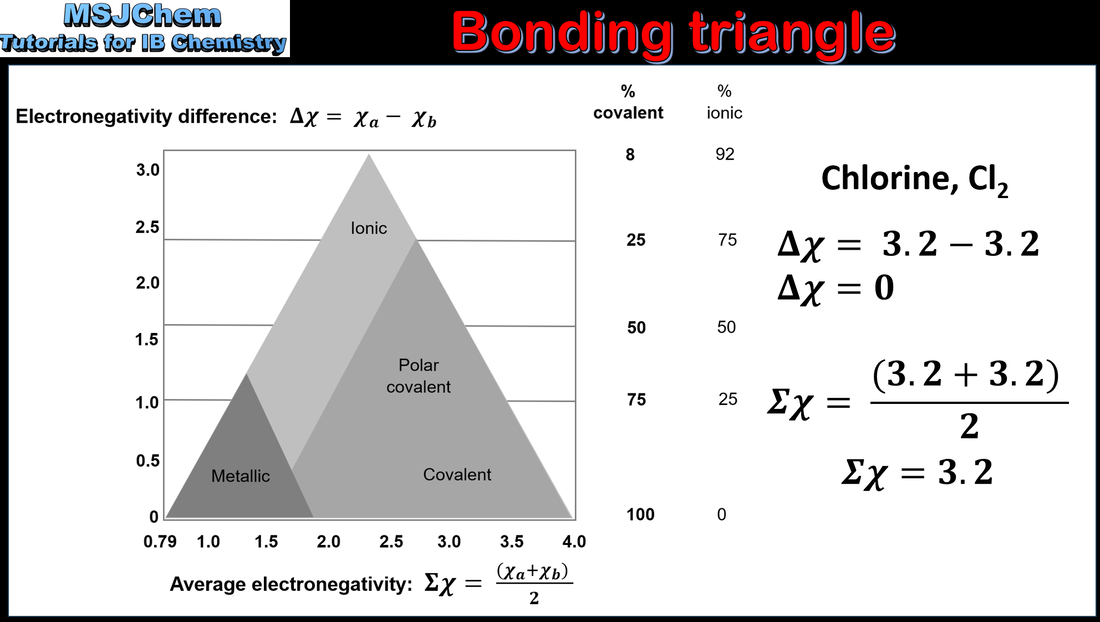
- Bonding is best described as a continuum between the ionic, covalent and metallic models, and can be represented by a bonding triangle.
- The position of a compound in the bonding triangle is determined by the relative contributions of the three bonding types to the overall bond.
- Use bonding models to explain the properties of a material.
- Determine the position of a compound in the bonding triangle from electronegativity data.
- Predict the properties of a compound based on its position in the bonding triangle.
- A triangular bonding diagram is provided in the data booklet.
- To illustrate the relationship between bonding type and properties, include example materials of varying percentage bonding character. Only binary compounds need to be considered.
- Calculations of percentage ionic character are not required.
- Electronegativity data are given in the data booklet.
- Structure 3.1 How do the trends in properties of period 3 oxides reflect the trend in their bonding?
- Structures 2.1, 2.2 What are the limitations of discrete bonding categories?
- Structure 2.1, 2.2, 2.3 Why do composites like reinforced concretes, which are made from ionic and covalently bonded components and steel bars, have unique properties?
Structure 2.4.3
Understandings:
Understandings:
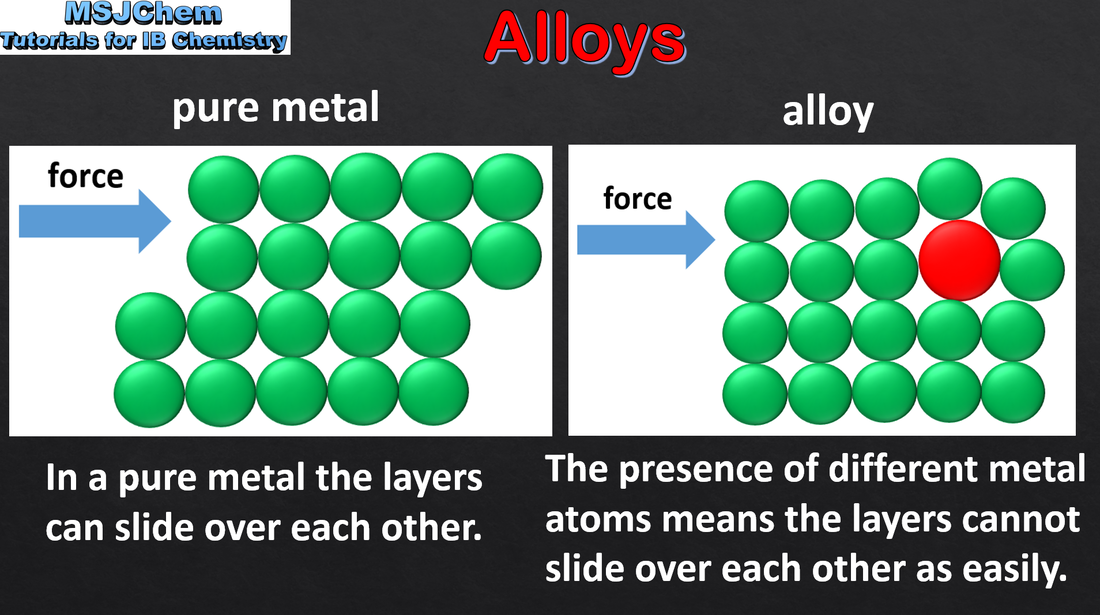
- Alloys are mixtures of a metal and other metals or non-metals. They have enhanced properties.
- Explain the properties of alloys in terms of non-directional bonding.
- Illustrate with common examples such as bronze, brass and stainless steel. Specific examples of alloys do not have to be learned.
- Structure 1.1 Why are alloys more correctly described as mixtures rather than as compounds?
Structure 2.4.4
Understandings:
Understandings:
- Polymers are large molecules, or macromolecules, made from repeating subunits called monomers.
- Describe the common properties of plastics in terms of their structure.
- Examples of natural and synthetic polymers should be discussed.
- Structure 3.2 What are the structural features of some plastics that make them biodegradable?
Video coming soon
Structure 2.4.5
Understandings:
Understandings:
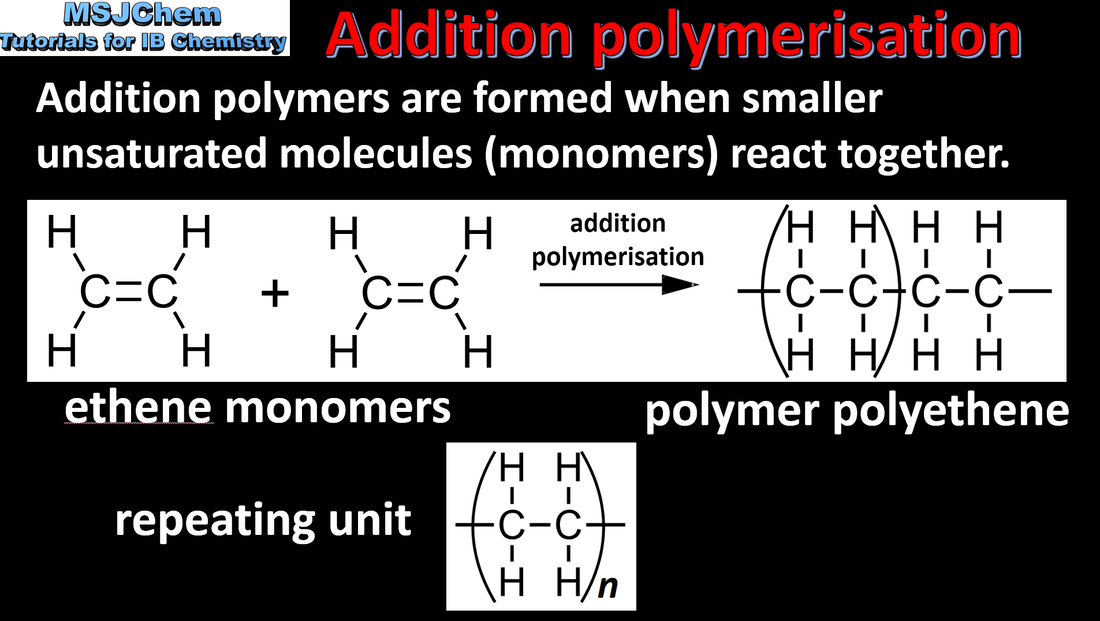
- Addition polymers form by the breaking of a double bond in each monomer.
- Represent the repeating unit of an addition polymer from given monomer structures.
- Examples should include polymerisation reactions of alkenes.
- Structures of monomers do not have to be learned but will be provided or will need to be deduced from the polymer.
- Structure 3.2 What functional groups in molecules can enable them to act as monomers for addition reactions?
- Reactivity 2.1 Why is the atom economy 100% for an addition polymerisation reaction?
HL content (2.4.6 only)
Structure 2.4.6
Understandings:
Understandings:
- Condensation polymers form by the reaction between functional groups in each monomer with the release of a small molecule.
- Represent the repeating unit of polyamides and polyesters from given monomer structures.
- All biological macromolecules form by condensation reactions and break down by hydrolysis.
- Structure 3.2 What functional groups in molecules can enable them to act as monomers for condensation reactions?
|
|
This video covers condensation polymers.
|
|
|
This video covers condensation polymers.
|
|
|
This video coves the formation of kevlar.
|