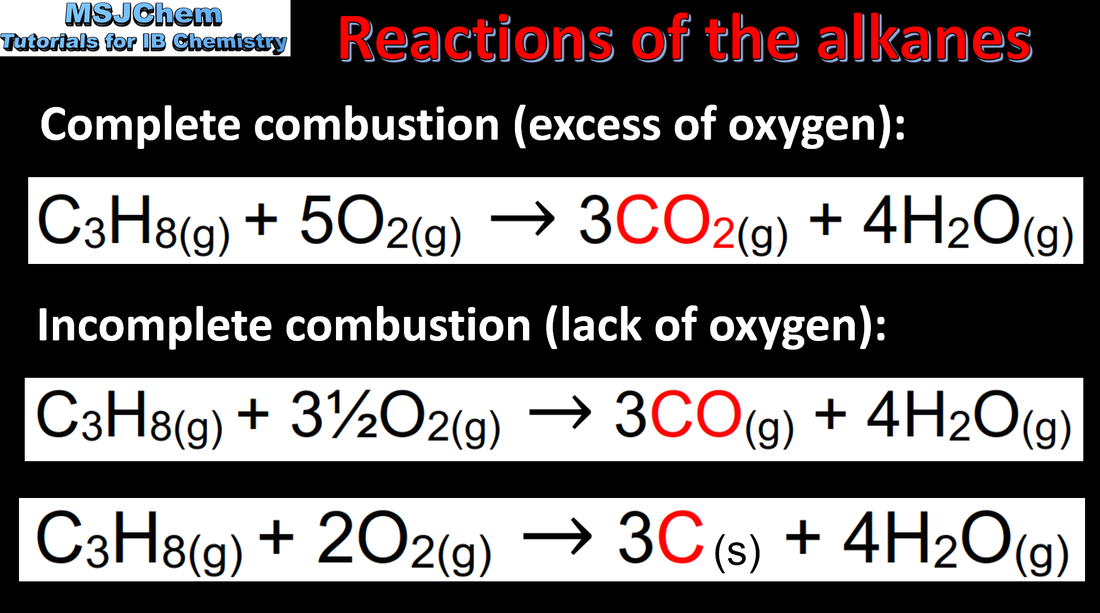Help support my work by joining the Member's Area or by becoming a Patron.
Topic 10 Organic chemistry
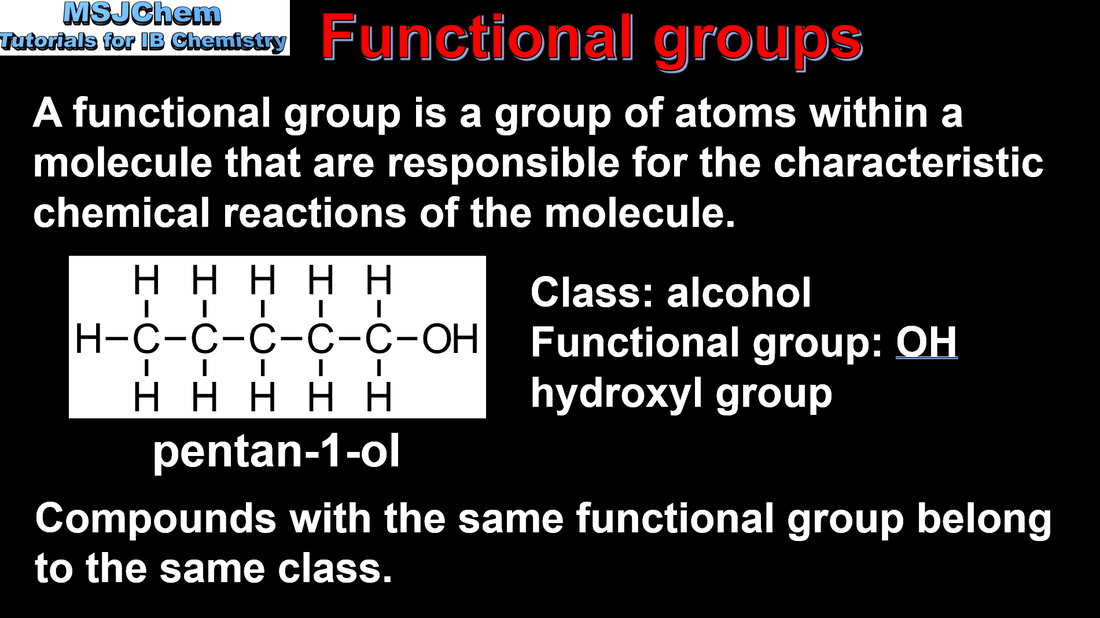
10.1 Functional groups
|
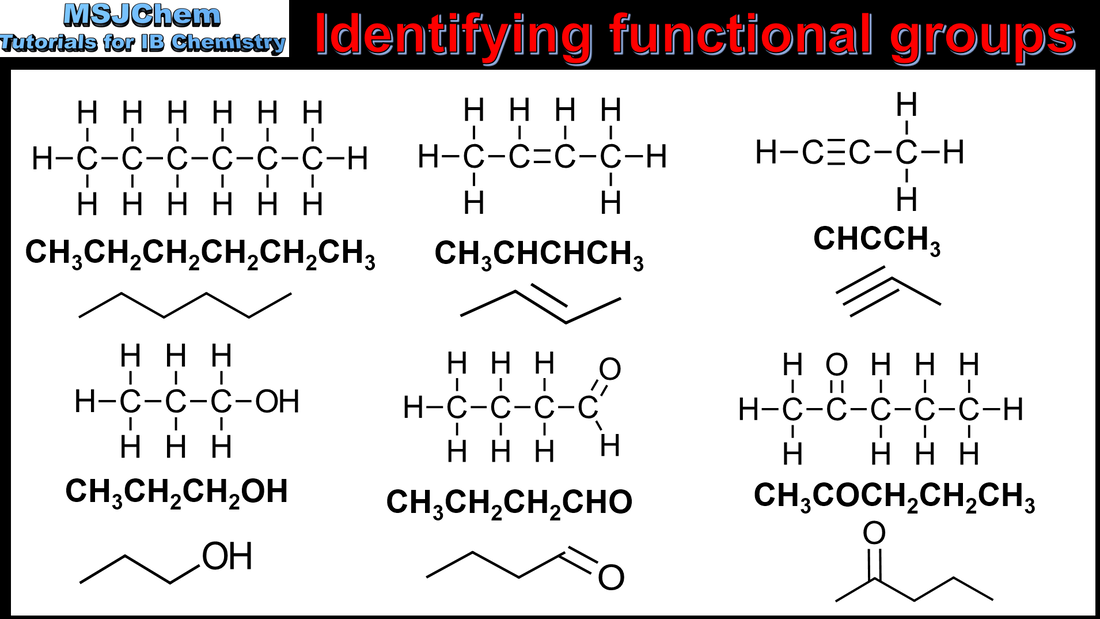
Identifying functional groups
|
Applications and skills:
Identification of different classes: alkanes, alkenes, alkynes, halogenoalkanes, alcohols, ethers, aldehydes, ketones, esters, carboxylic acids, amines, amides, nitriles and arenes. Identification of typical functional groups in molecules eg phenyl, hydroxyl, carbonyl, carboxyl, carboxamide, aldehyde, ester, ether, amine, nitrile, alkyl, alkenyl and alkynyl. Guidance: The distinction between class names and functional group names needs to be made. Eg for OH, hydroxyl is the functional group whereas alcohol is the class name. It should be noted that the functional group in a ketone can also be referred to as a carbonyl function. |
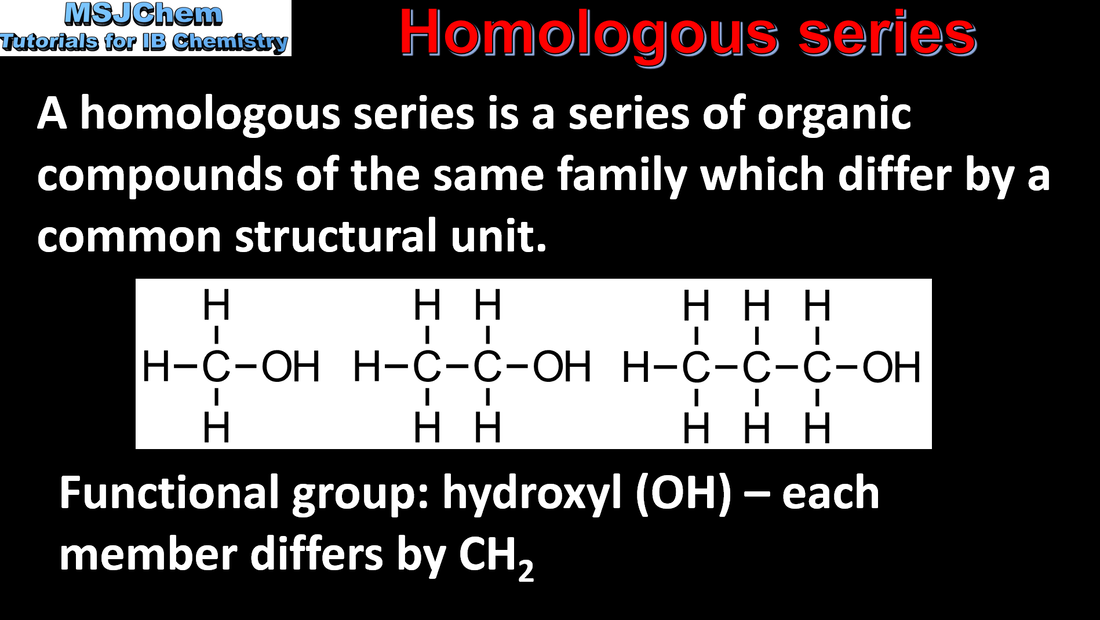
10.1 Homologous series
10.1 Classification of organic compounds
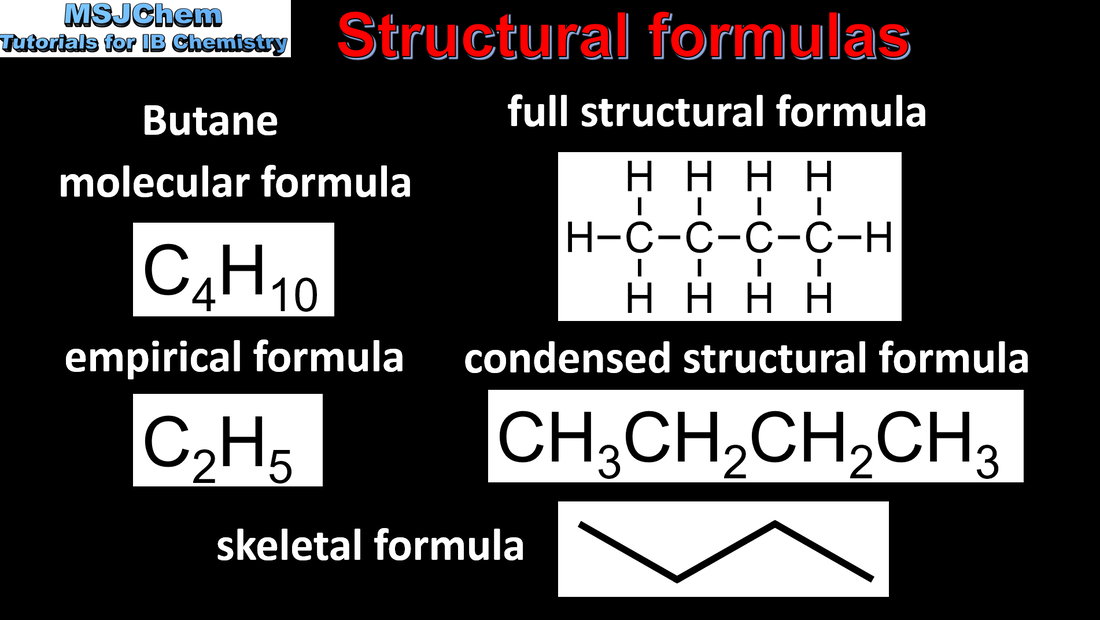
10.1 Structural formulas
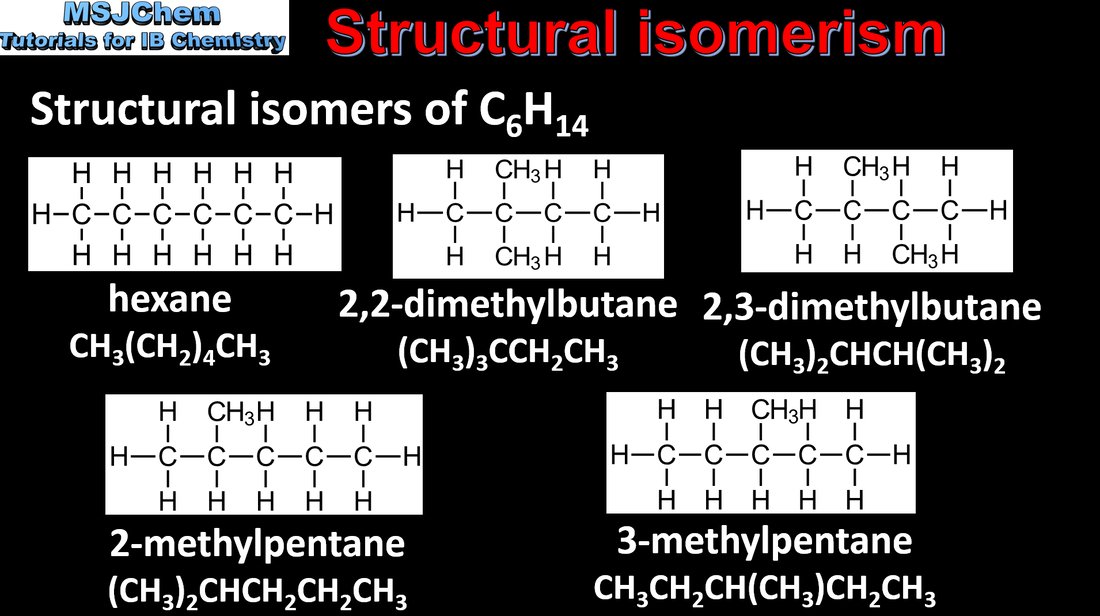
10.1 Structural isomers
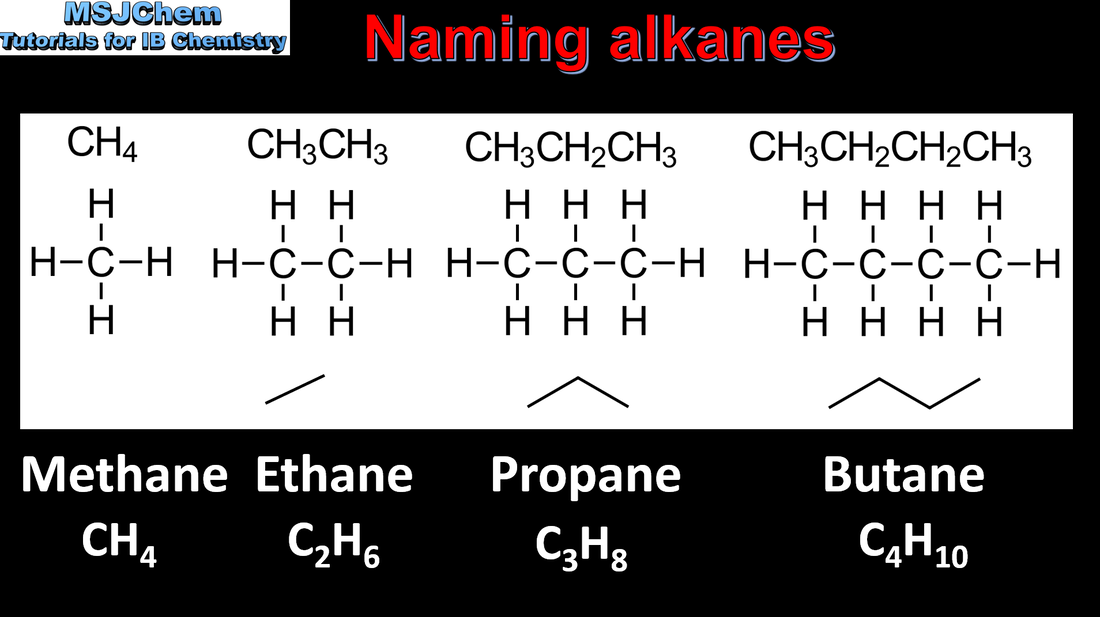
10.1 Naming alkanes (straight-chain and cyclic alkanes)
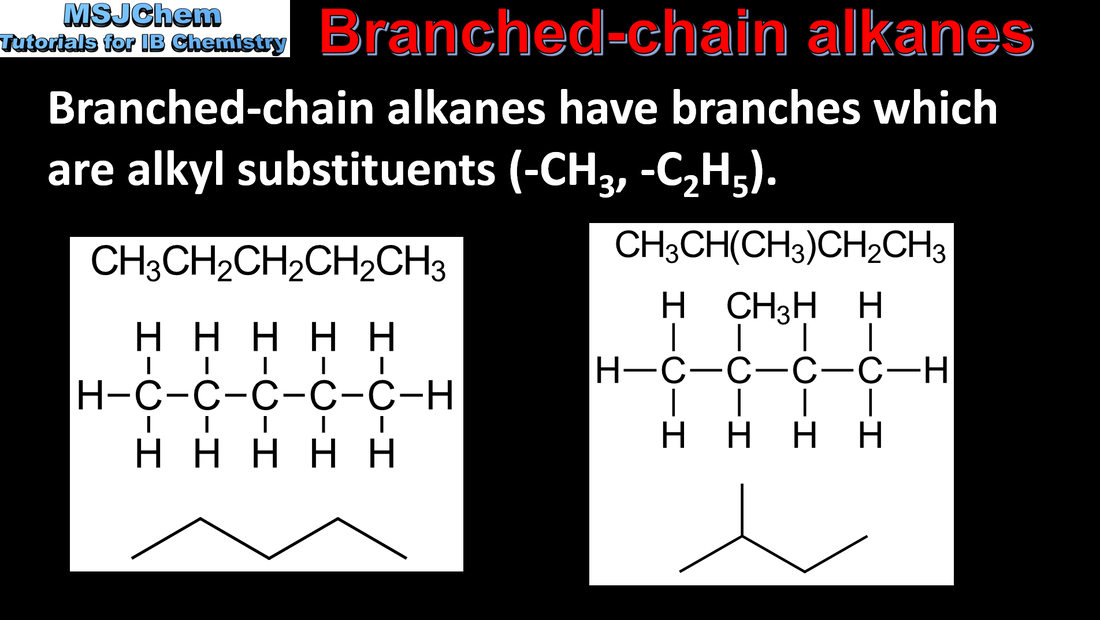
10.1 Naming branched-chain alkanes
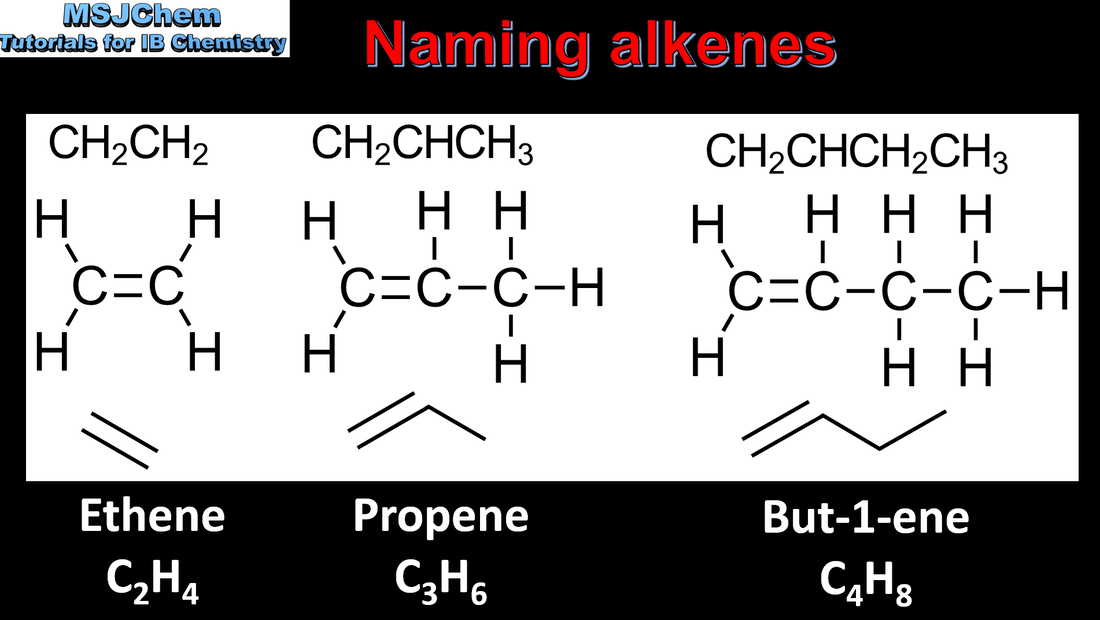
10.1 Naming alkenes
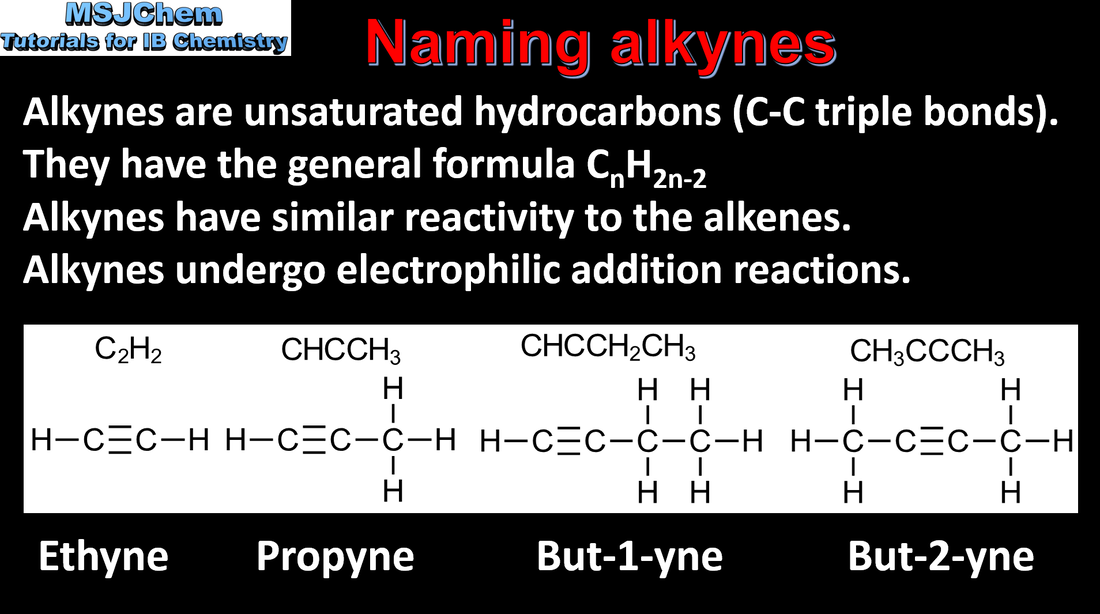
10.1 Naming alkynes
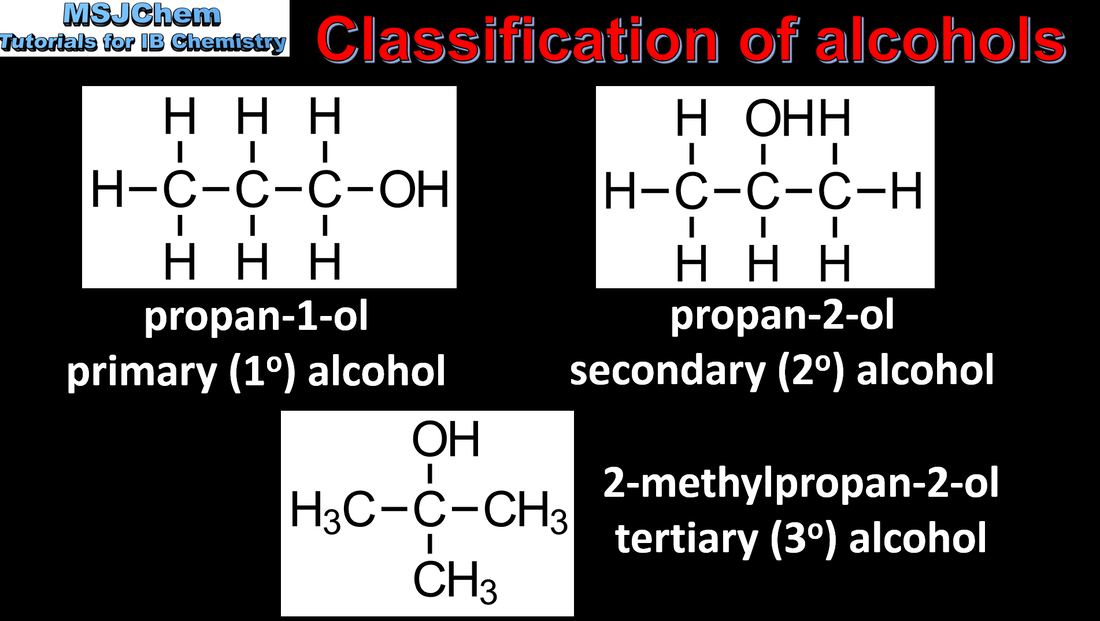
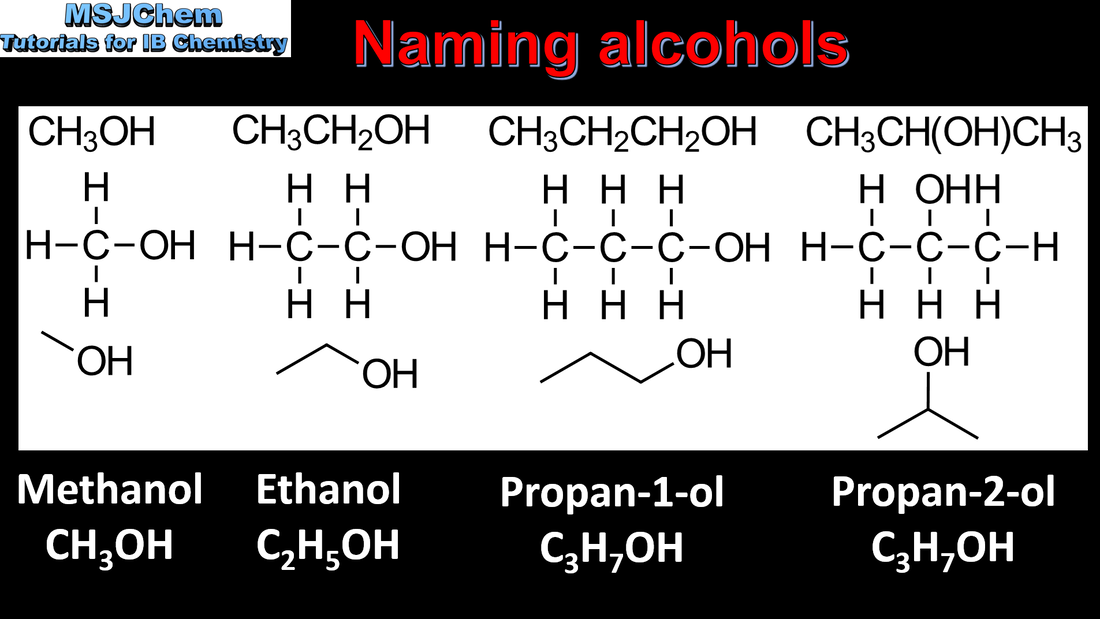
10.1 Naming alcohols
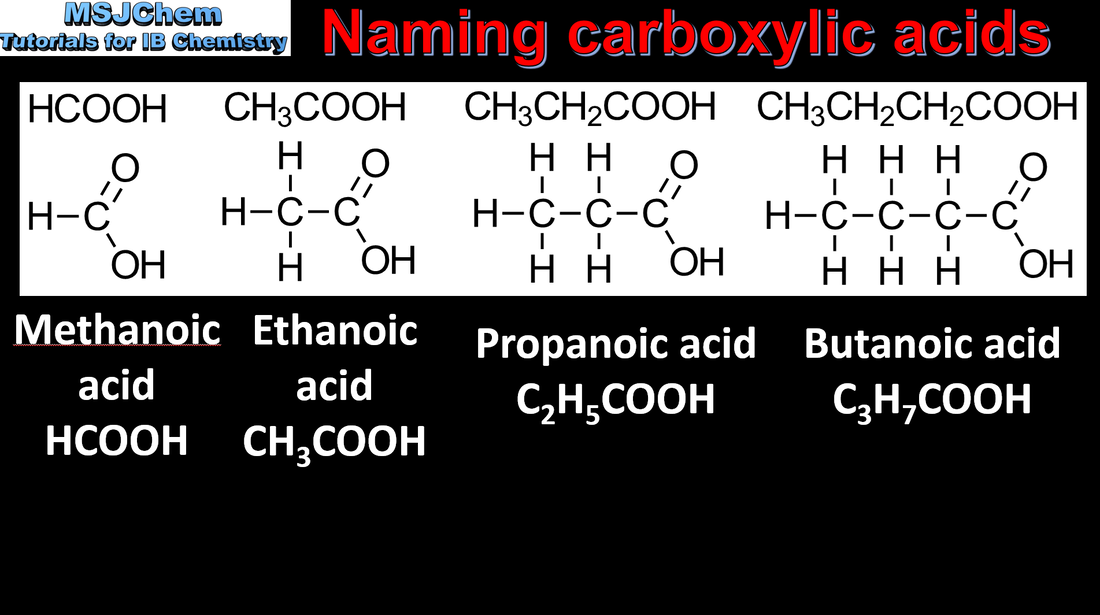
10.1 Naming carboxylic acids
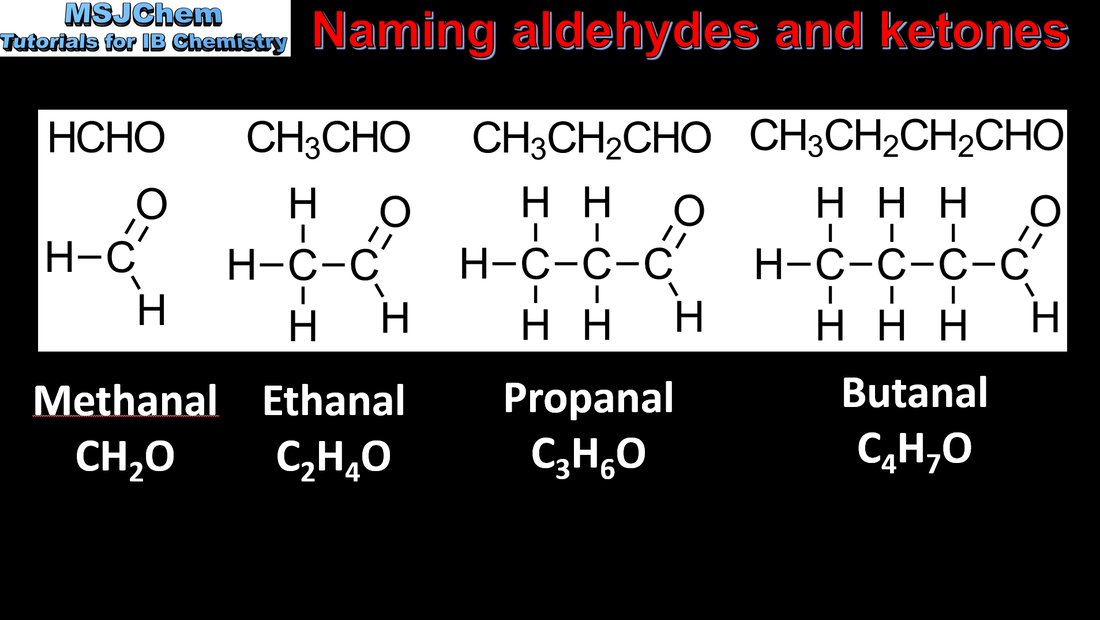
10.1 Naming aldehydes and ketones
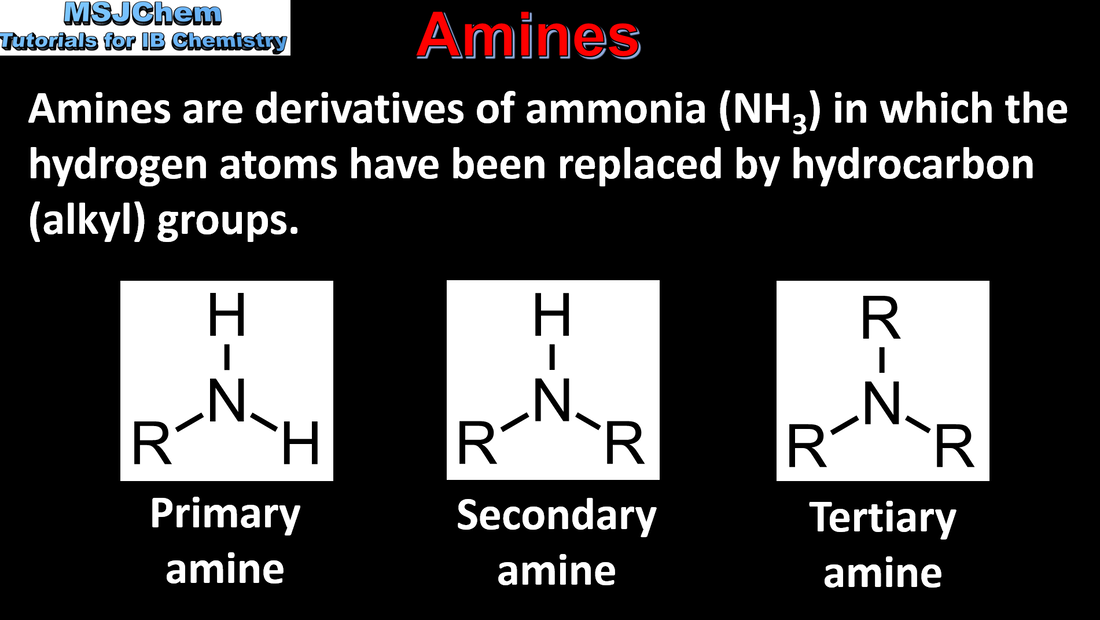
10.1 Naming amines
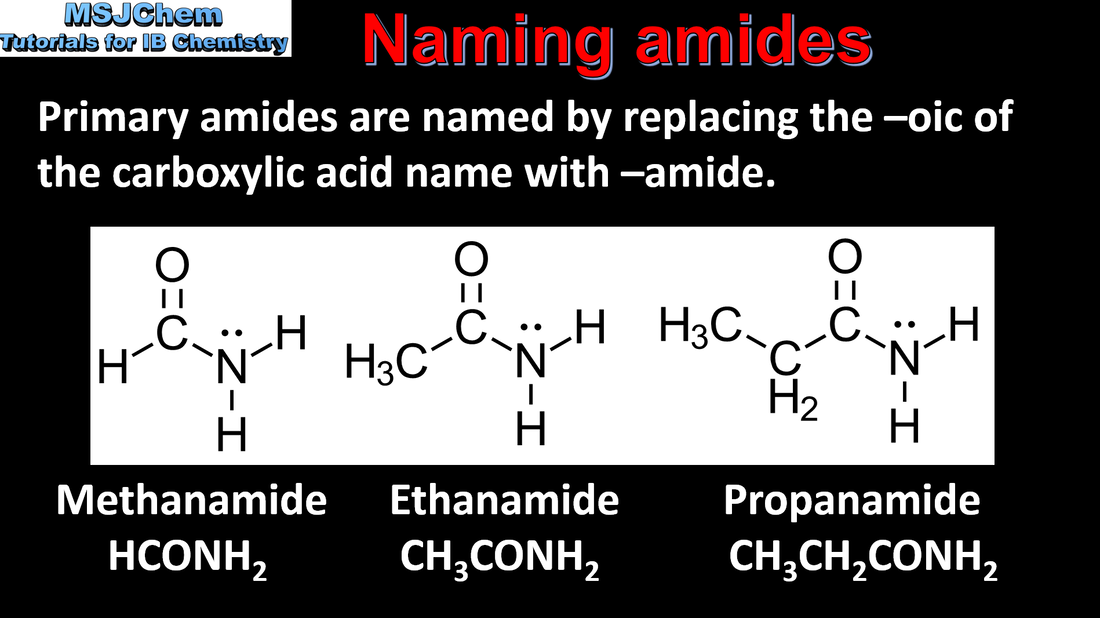
10.1 Naming amides
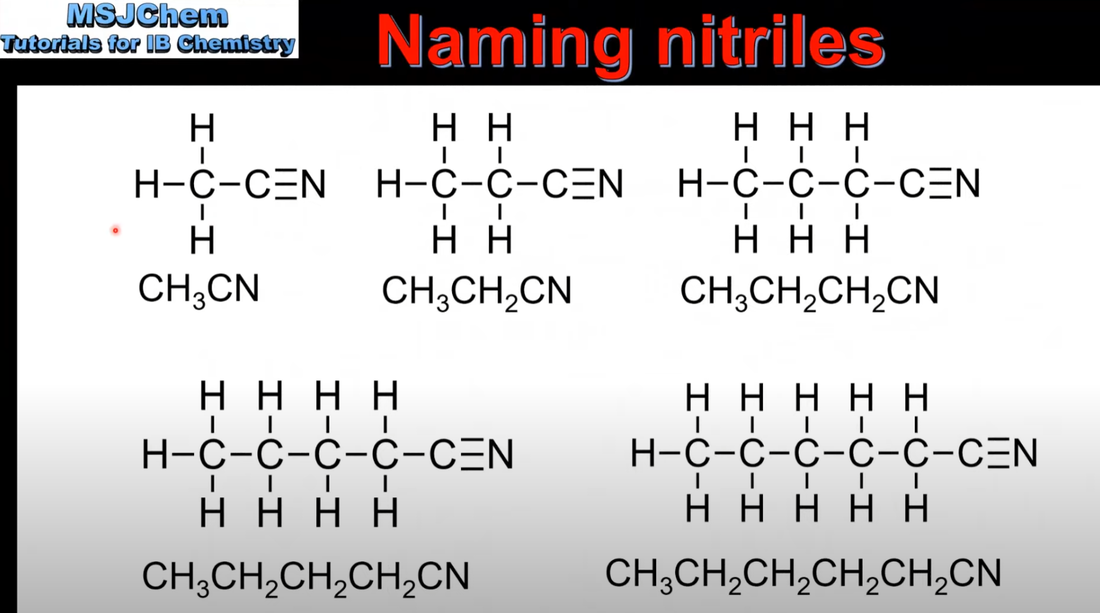
10.1 Naming nitriles
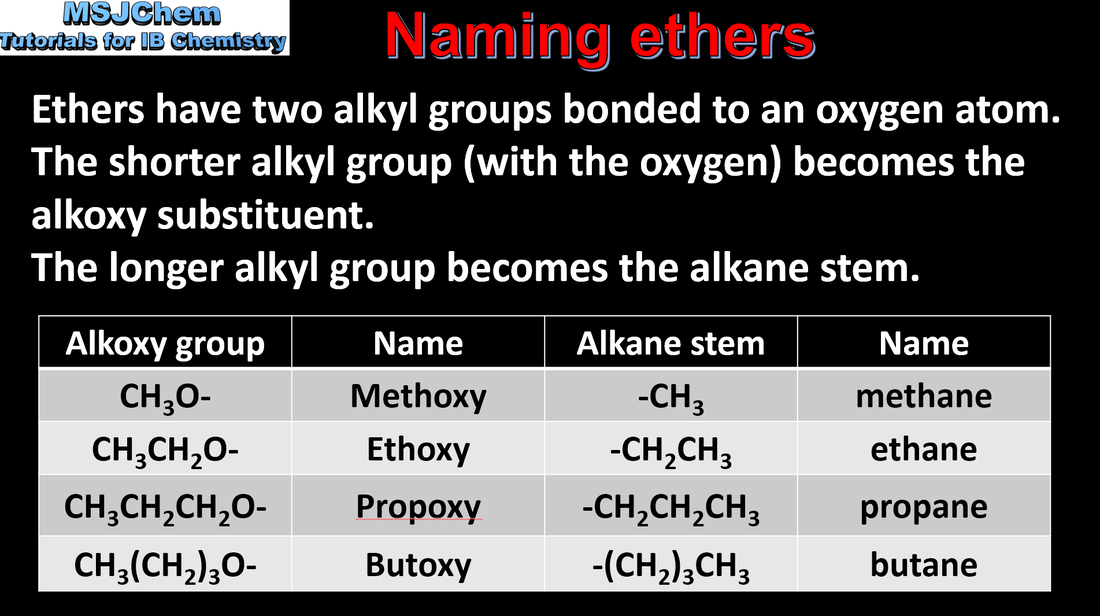
10.1 Naming ethers
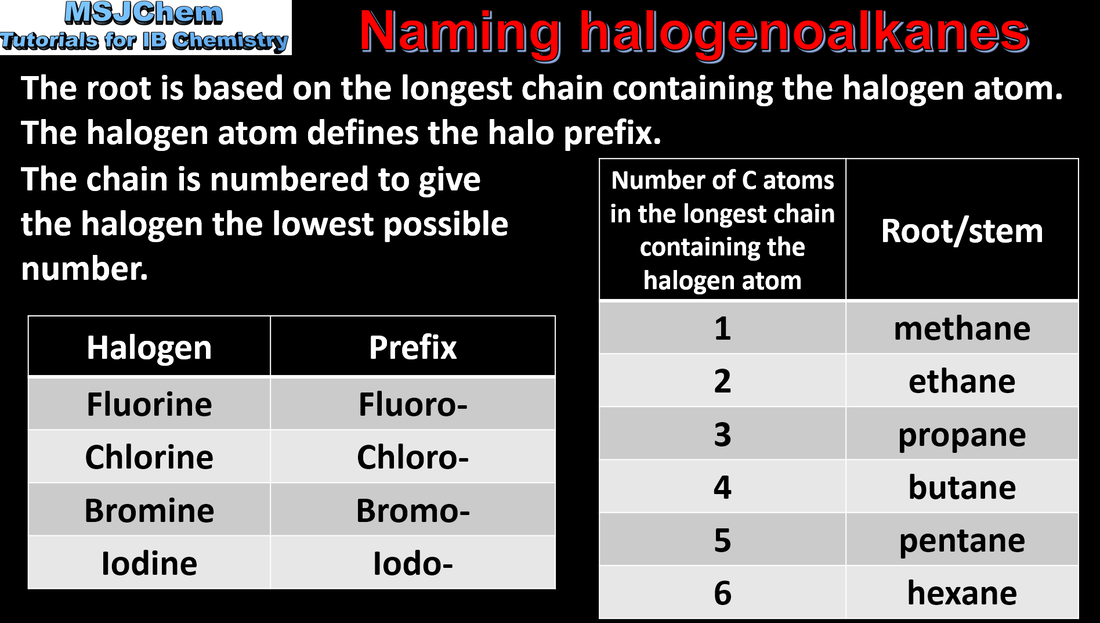
10.1 Naming halogenoalkanes
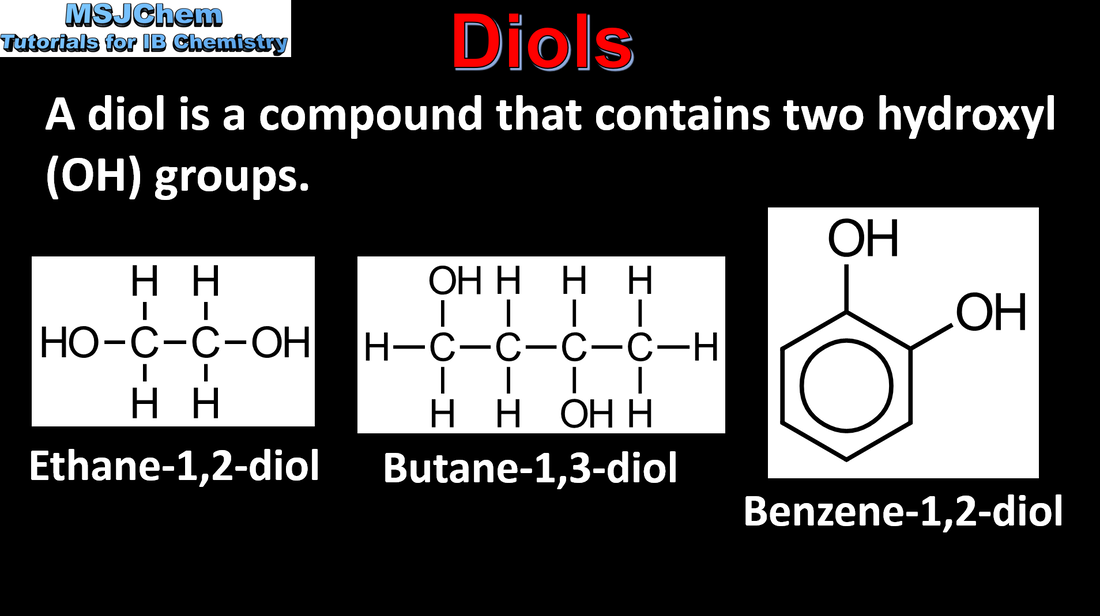
10.1 Diols and dicarboxylic acids
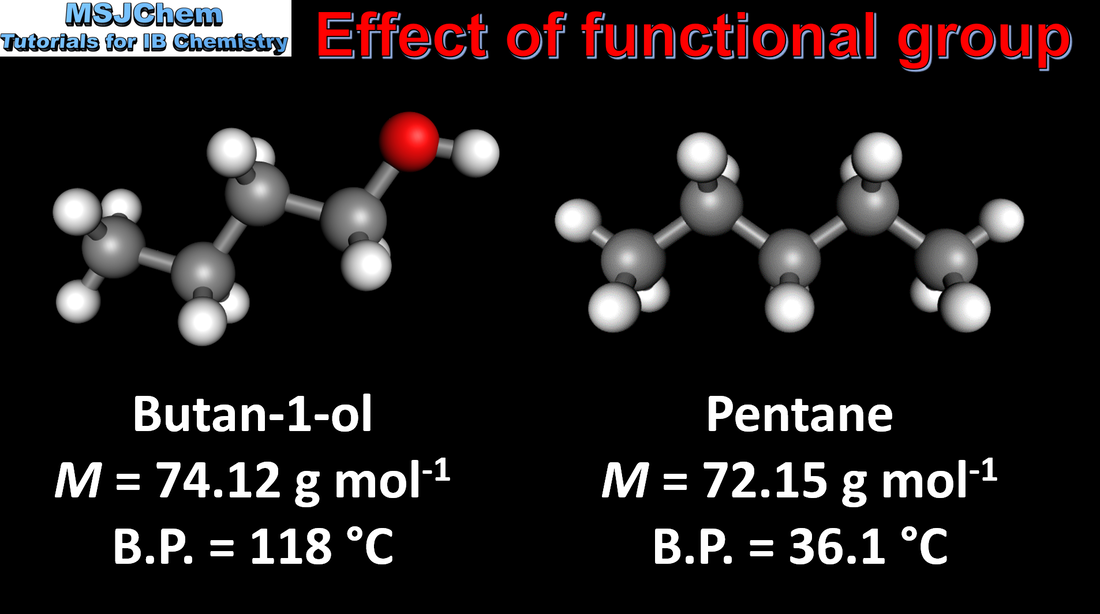
10.1 Boiling points of organic compounds
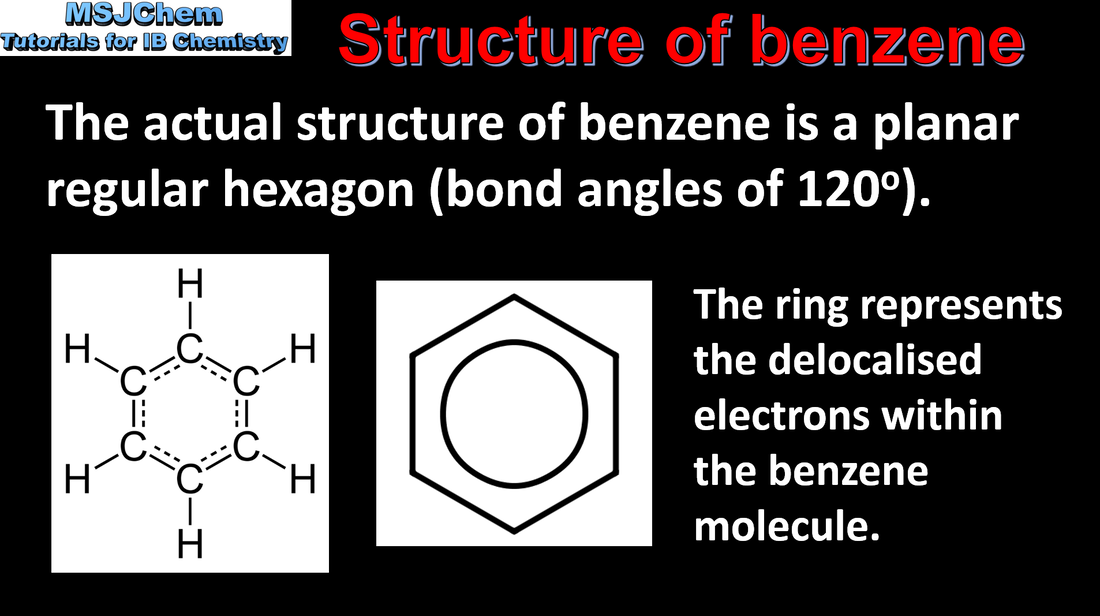
10.1 Structure of benzene
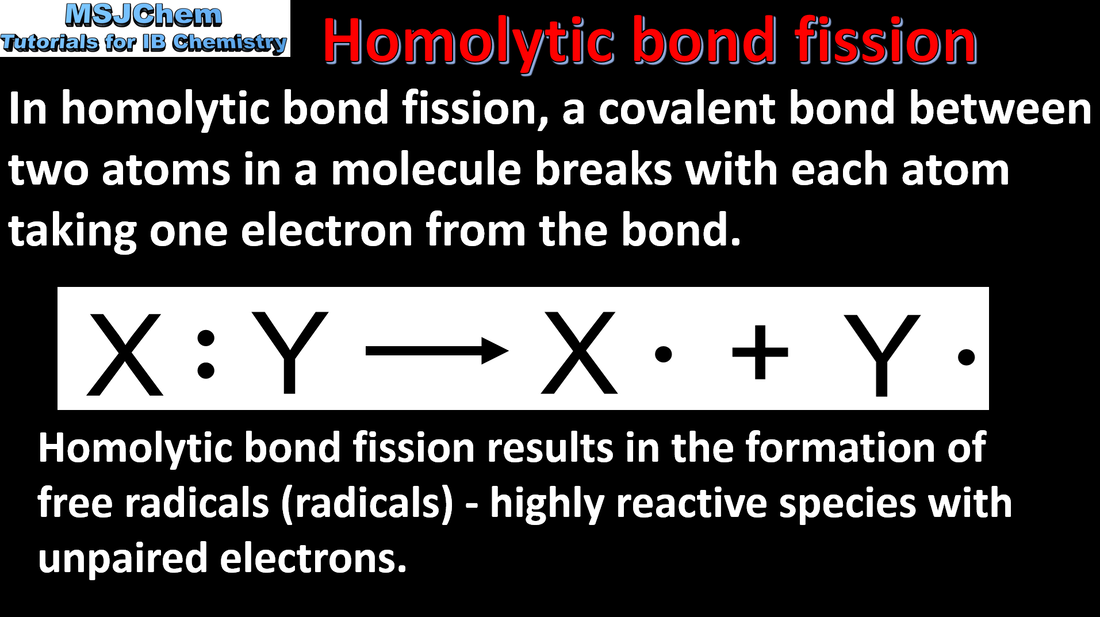
10.2 Homolytic and heterolytic bond fission
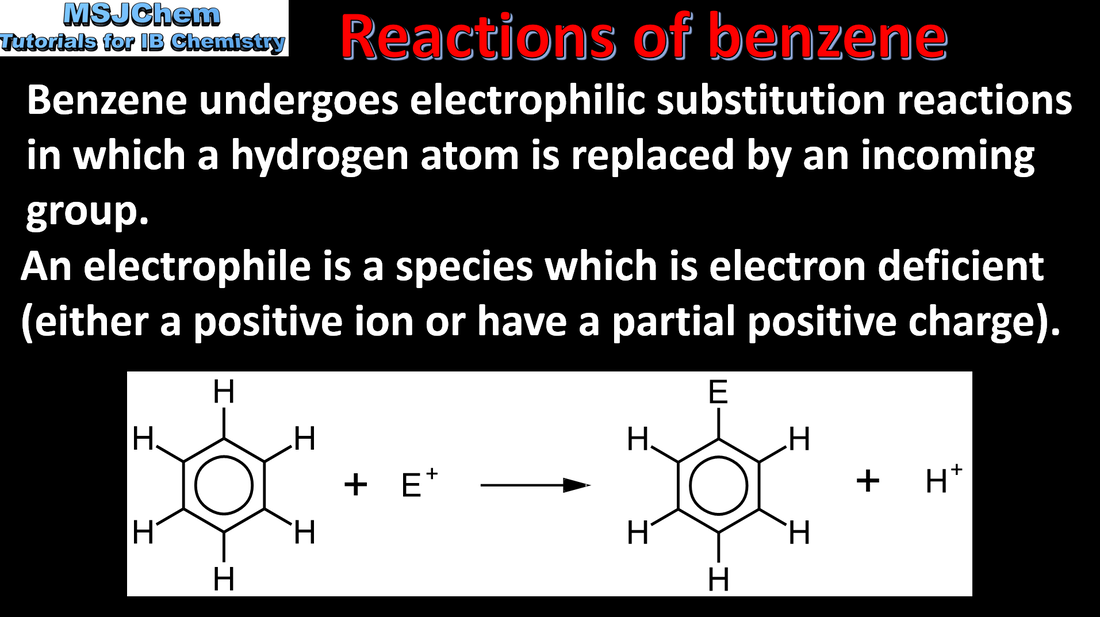
10.2 Reactions of benzene
10.2 Combustion reactions of the alkanes
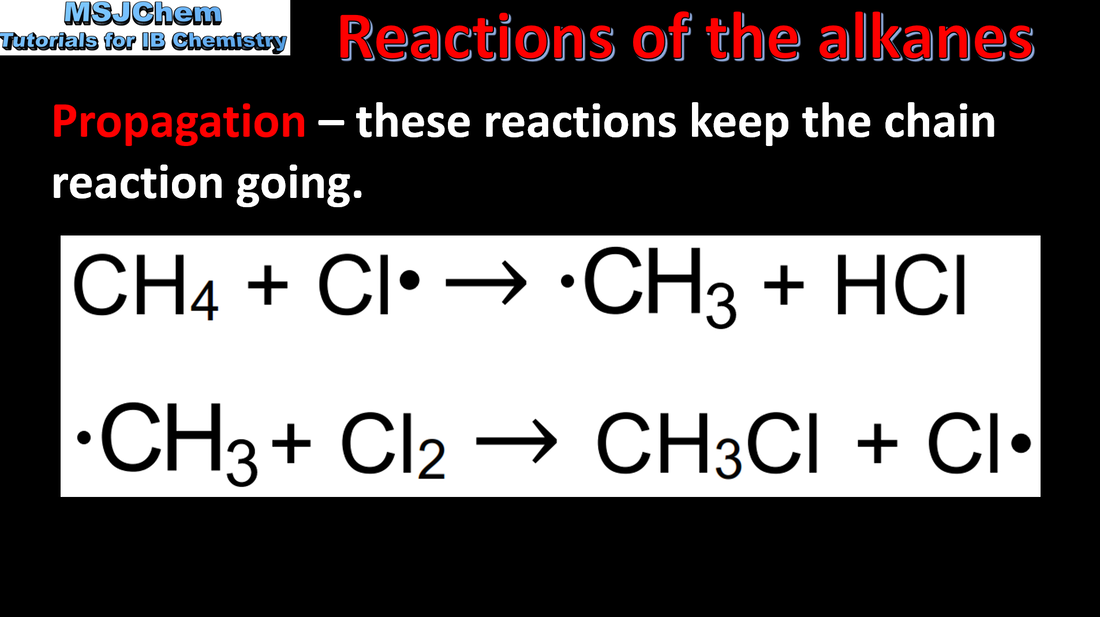
10.2 Free radical substitution reactions of the alkanes
|
Understandings:
Alkanes have low reactivity and undergo free-radical substitution reactions. Applications and skills: Explanation of the reaction of methane and ethane with halogens in terms of a free-radical substitution mechanism involving photochemical homolytic fission. Guidance: Reference should be made to initiation, propagation and termination steps in free-radical substitution reactions. Free radicals should be represented by a single dot. |
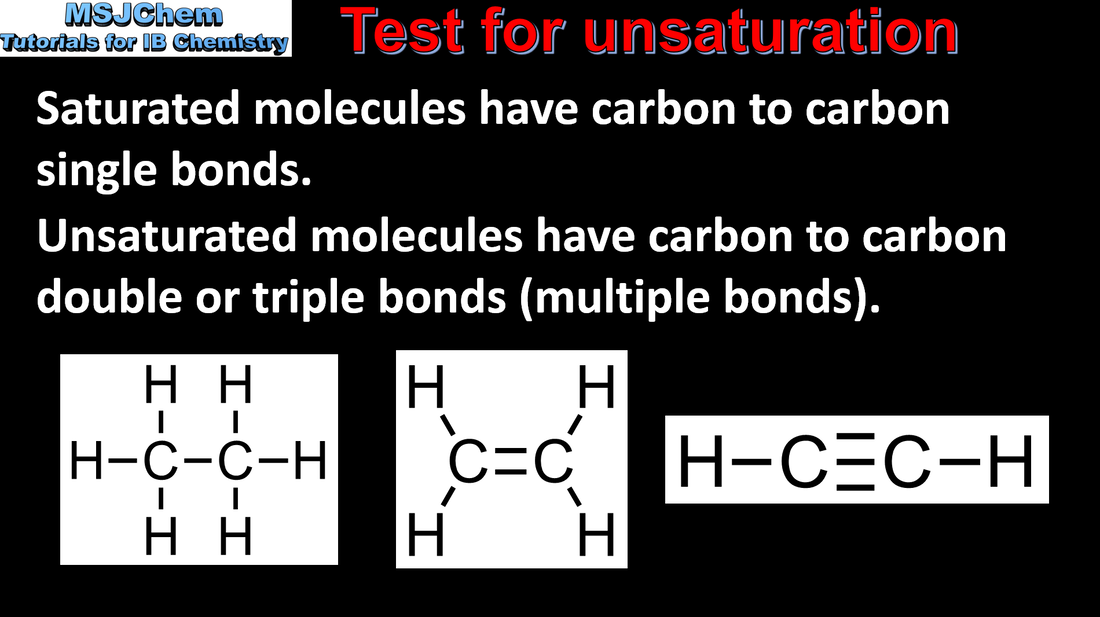
10.2 Test for unsaturation
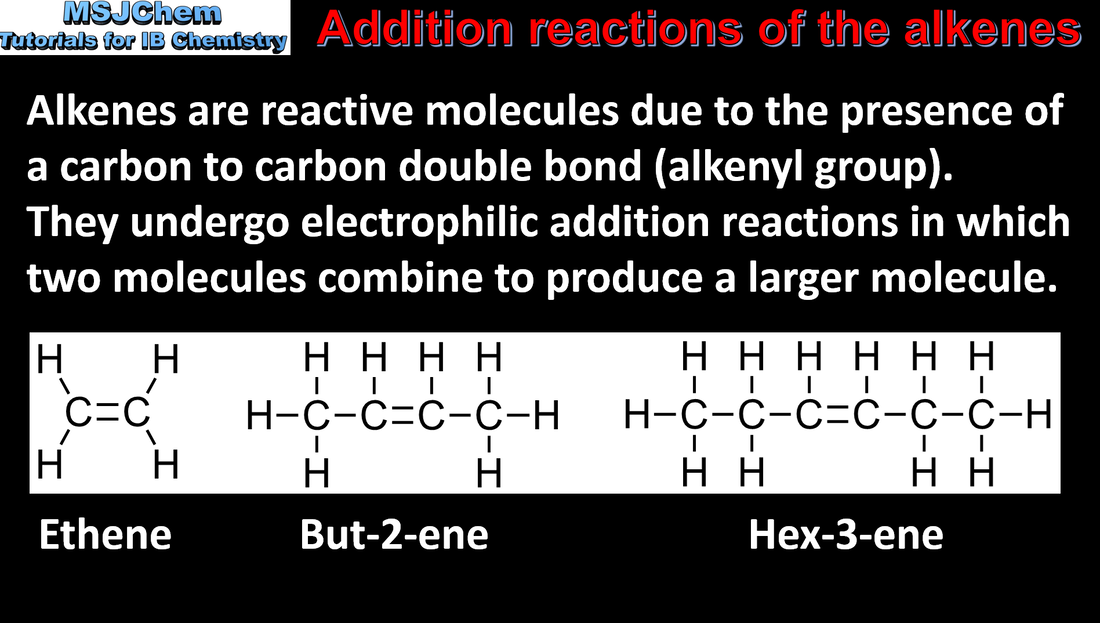
10.2 Addition reactions of the alkenes
|
Understandings:
Alkenes are more reactive than alkanes and undergo addition reactions Application and skills: Writing equations for the reactions of alkenes with hydrogen and halogens and of symmetrical alkenes with hydrogen halides and water. Note that the conditions for the hydrogenation of alkenes include a nickel catalyst and high pressure and temperature.
|
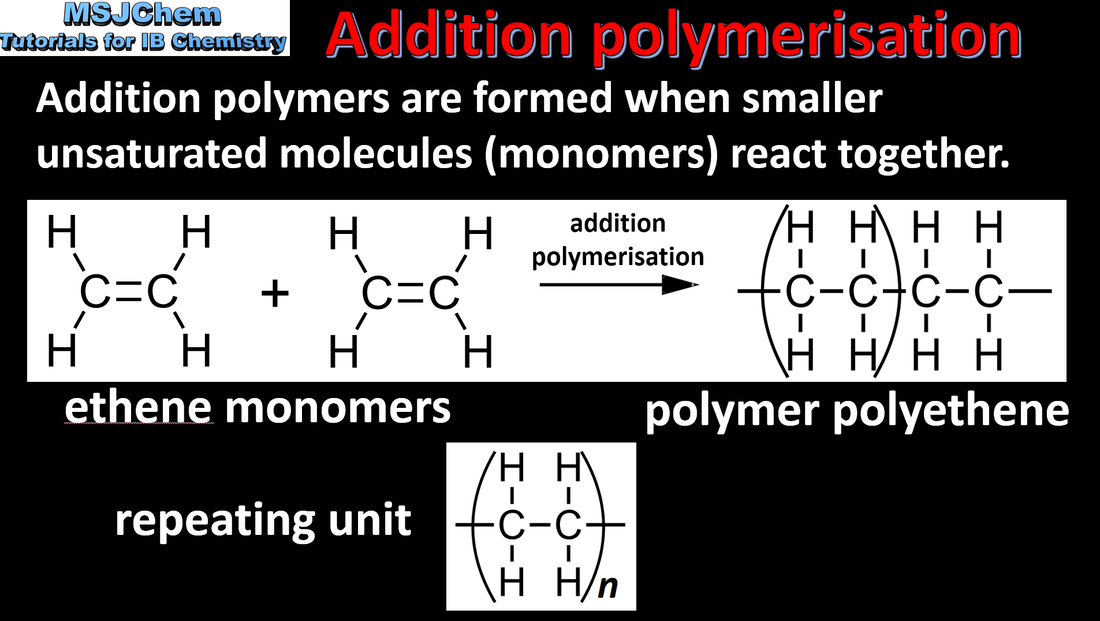
10.2 Addition polymerisation
|
Applications and skills:
Outline of the addition polymerisation of alkenes. Relationship between the structure of the monomer to the polymer and repeating unit. |
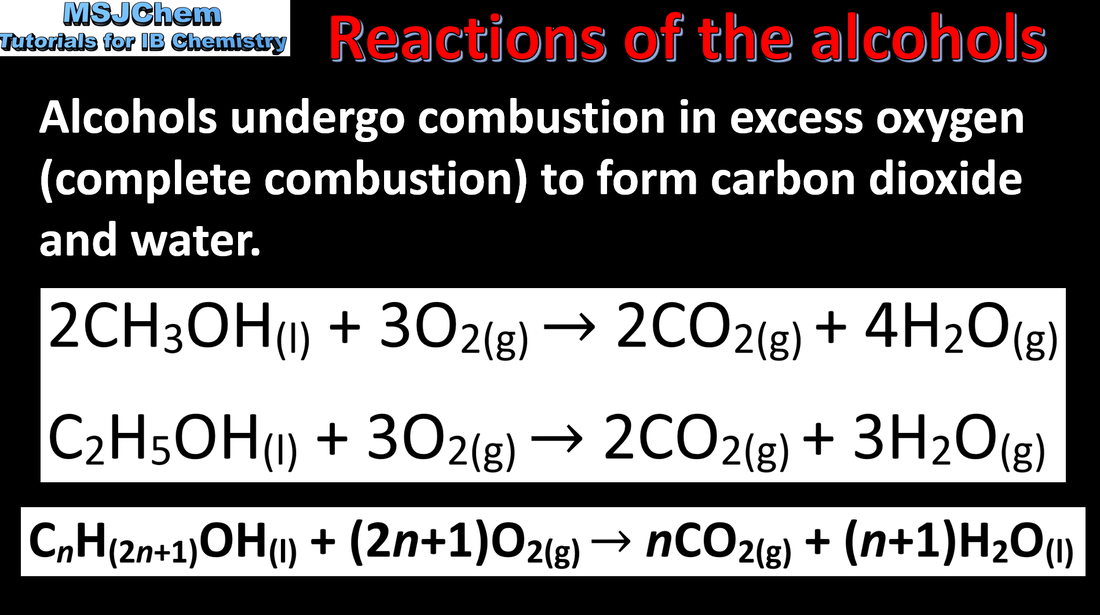
10.2 Combustion reactions of the alcohols
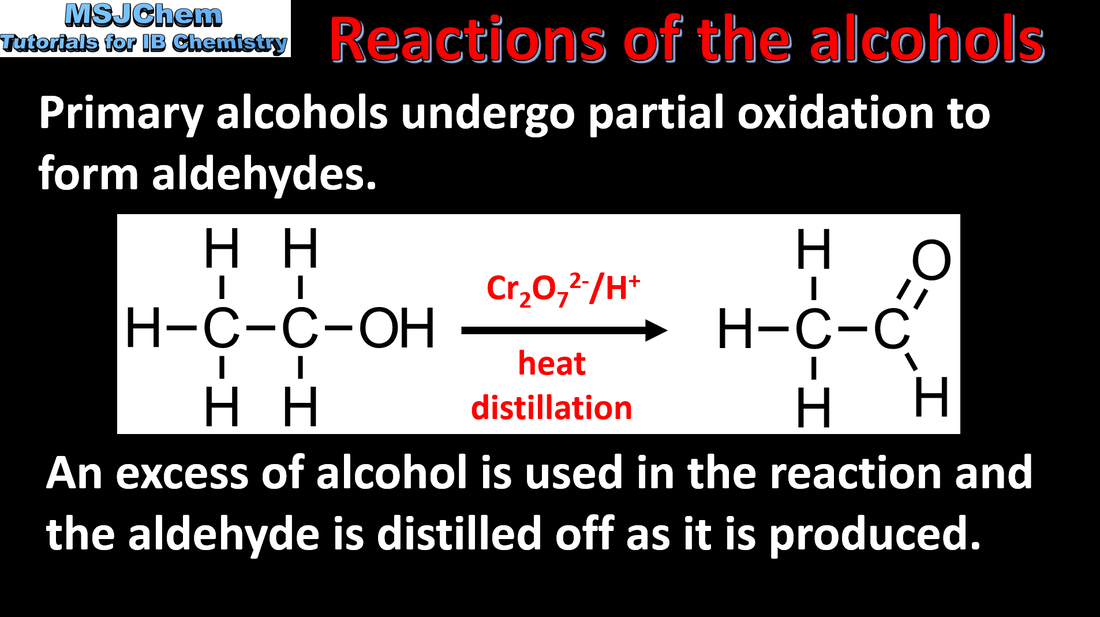
10.2 Reactions of the alcohols (oxidation)
|
Understandings:
Some alcohols undergo oxidation reactions. Applications and skills: Writing equations for the oxidation reactions of primary and secondary alcohols (using acidified potassium dichromate(VI) or potassium manganate(VII) as oxidizing agents). Explanation of distillation and reflux in the isolation of the aldehyde and carboxylic acid products. |
The aldehyde can be distilled off because it has a lower boiling point than the alcohol or carboxylic acid (aldehydes do not form hydrogen bonds between molecules). If the aldehyde is left in contact with the oxidising agent, then the carboxylic acid is formed. An alternative oxidising agent is acidified potassium manganate(VII) solution which changes colour from purple to colourless.
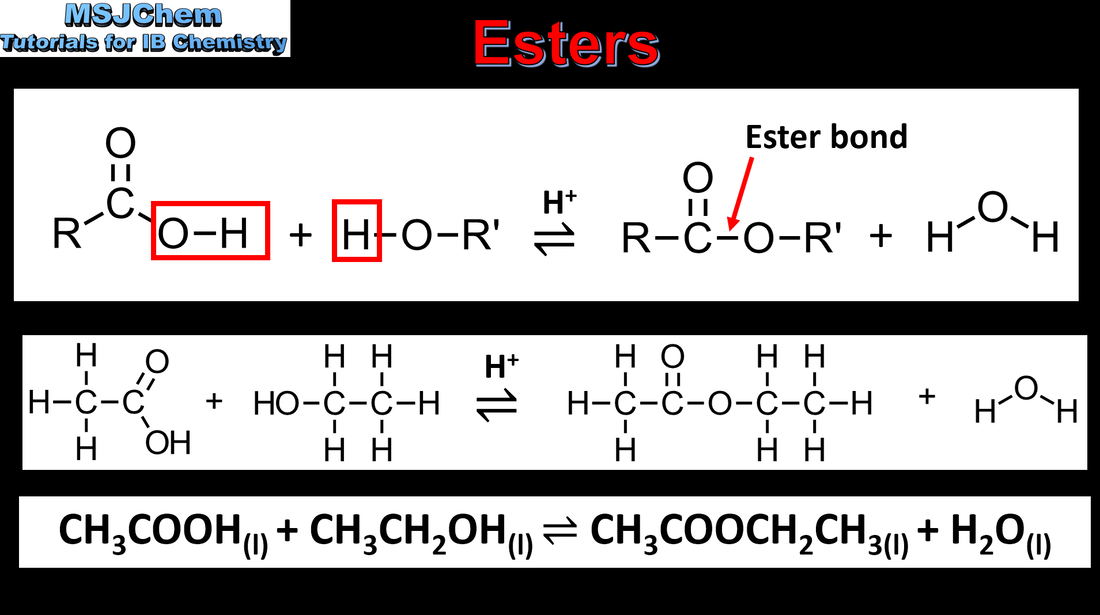
10.2 Esterification/condensation reactions of alcohols and carboxylic acids
|
Understandings
Alcohols undergo nucleophilic substitution reactions with acids (also called esterification or condensation) Applications and skills: Writing the equation for the condensation reaction of an alcohol with a carboxylic acid, in the presence of a catalyst (eg concentrated sulfuric acid) to form an ester. |
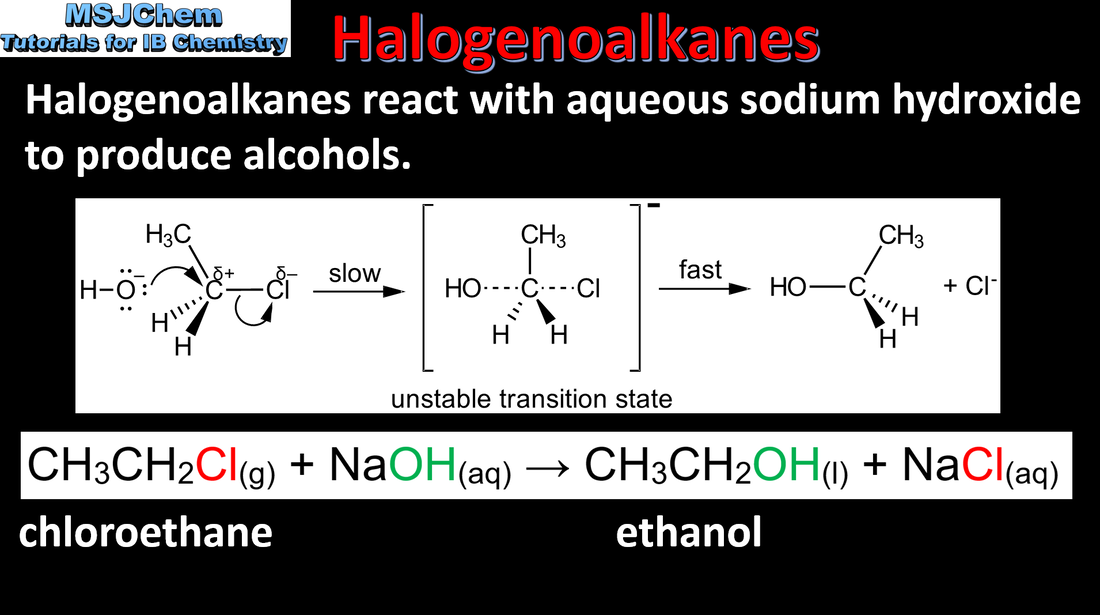
10.2 Reactions of the halogenoalkanes
|
Understandings:
Halogenoalkanes are more reactive than alkanes. They can undergo (nucleophilic) substitution reactions. A nucleophile is an electron-rich species containing a lone pair that it donates to an electron-deficient carbon. Applications and skills: Writing the equation for the substitution reactions of halogenoalkanes with aqueous sodium hydroxide. Guidance: The mechanisms of SN1 and SN2 and electrophilic substitution reactions are not required. |